
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), an N-acylethanolamine (NAE), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of taste, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.

Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge, a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria. It is a potent functional analog of capsaicin, the active ingredient in chili peppers.

TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.
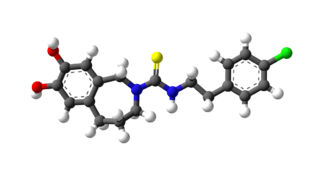
Capsazepine is a synthetic antagonist of capsaicin. It is used as a biochemical tool in the study of TRPV ion channels.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.

Transient receptor potential cation channel, subfamily A, member 1, also known as transient receptor potential ankyrin 1, TRPA1, or The Wasabi Receptor, is a protein that in humans is encoded by the TRPA1 gene.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily V member 4 is an ion channel protein that in humans is encoded by the TRPV4 gene.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.
Transient receptor potential cation channel, subfamily V, member 3, also known as TRPV3, is a human gene encoding the protein of the same name.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.
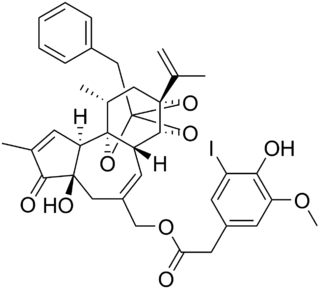
Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.
Vanillotoxins are neurotoxins found in the venom of the tarantula Psalmopoeus cambridgei. They act as agonists for the transient receptor potential cation channel subfamily V member 1 (TRPV1), activating the pain sensory system. VaTx1 and 2 also act as antagonists for the Kv2-type voltage-gated potassium channel (Kv2), inducing paralytic behavior in small animals.
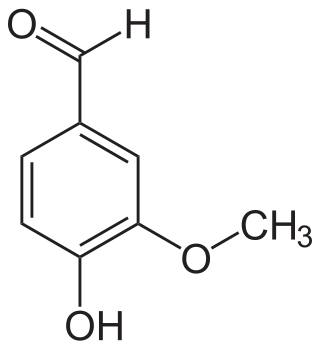
The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.
RhTx is a small peptide toxin from Scolopendra subspinipes mutilans, also called the Chinese red-headed centipede. RhTx binds to the outer pore region of the temperature regulated TRPV1 ion channel, preferably in activated state, causing a downwards shift in the activation threshold temperature, which leads to the immediate onset of heat pain.
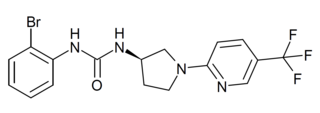
SB-705498 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It has been evaluated in clinical trials for the treatment of rhinitis and chronic cough.

Double-knot toxin (DkTx), also known as Tau-theraphotoxin-Hs1a or Tau-TRTX-Hs1a, is a toxin found in the venom of the Chinese Bird spider, a tarantula species primarily living in the Guangxi province of China. This toxin, characterized by its bivalent structure of two Inhibitor Cysteine Knots (ICK), is thought to induce excruciating and long-lasting pain by activating the transient receptor potential vanilloid 1 (TRPV1) channel.





















