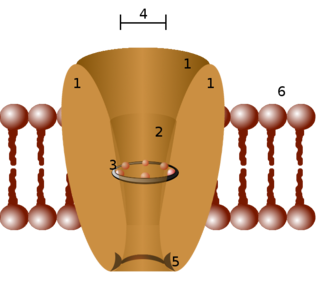
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of taste, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.
A calcium channel is an ion channel which shows selective permeability to calcium ions. It is sometimes synonymous with voltage-gated calcium channel, which are a type of calcium channel regulated by changes in membrane potential. Some calcium channels are regulated by the binding of a ligand. Other calcium channels can also be regulated by both voltage and ligands to provide precise control over ion flow. Some cation channels allow calcium as well as other cations to pass through the membrane.
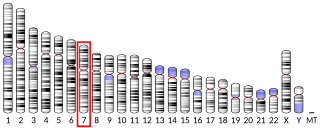
TRPV6 is a membrane calcium (Ca2+) channel protein which is particularly involved in the first step in Ca2+absorption in the intestine.
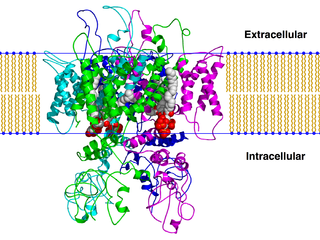
The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
TRPC is a family of transient receptor potential cation channels in animals.

TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.
TRPM is a family of transient receptor potential ion channels (M standing for wikt:melastatin). Functional TRPM channels are believed to form tetramers. The TRPM family consists of eight different channels, TRPM1–TRPM8.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.
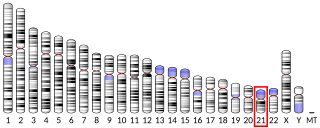
Transient receptor potential cation channel, subfamily M, member 2, also known as TRPM2, is a protein that in humans is encoded by the TRPM2 gene.

Transient receptor potential cation channel, subfamily A, member 1, also known as transient receptor potential ankyrin 1, TRPA1, or The Wasabi Receptor, is a protein that in humans is encoded by the TRPA1 gene.
Transient receptor potential cation channel subfamily M member 5 (TRPM5), also known as long transient receptor potential channel 5 is a protein that in humans is encoded by the TRPM5 gene.

Transient receptor potential cation channel subfamily V member 4 is an ion channel protein that in humans is encoded by the TRPV4 gene.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.
Transient receptor potential cation channel, subfamily V, member 3, also known as TRPV3, is a human gene encoding the protein of the same name.

Transient receptor potential cation channel subfamily V member 5 is a calcium channel protein that in humans is encoded by the TRPV5 gene.
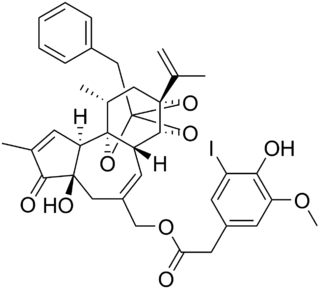
Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.
The transient receptor potential Ca2+ channel (TRP-CC) family (TC# 1.A.4) is a member of the voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology:
- the canonical or classic TRPs,
- the vanilloid receptor TRPs,
- the melastatin or long TRPs,
- ankyrin (whose only member is the transmembrane protein 1 [TRPA1])
- TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins,
- the polycystins
- and mucolipins.
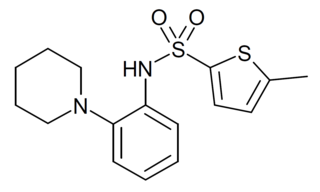
MK6-83 is a chemical compound which acts as a channel opener for the TRPML family of calcium channels, with moderate selectivity for TRPML1 over the related TRPML2 and TRPML3 subtypes.






















