The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

Inflammation is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. The five cardinal signs are heat, pain, redness, swelling, and loss of function.

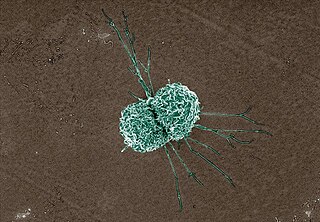
Neutrophils are a type of phagocytic white blood cell and part of innate immunity. More specifically, they form the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. Their functions vary in different animals. They are also known as neutrocytes, heterophils or polymorphonuclear leukocytes.

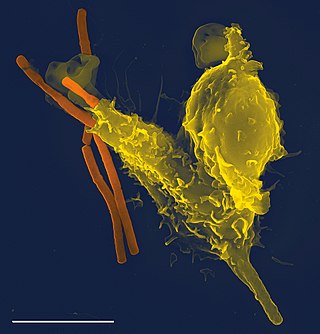
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae. Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery. Phagocytes occur in many species; some amoebae behave like macrophage phagocytes, which suggests that phagocytes appeared early in the evolution of life.

Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also influence adaptive immune responses and exert tissue repair functions. There are at least three subclasses of monocytes in human blood based on their phenotypic receptors.

Granulocytes are cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear, that is, they have varying shapes (morphology) of the nucleus ; and are referred to as polymorphonuclear leukocytes. In common terms, polymorphonuclear granulocyte refers specifically to "neutrophil granulocytes", the most abundant of the granulocytes; the other types have varying morphology. Granulocytes are produced via granulopoiesis in the bone marrow.

Cellular immunity, also known as cell-mediated immunity, is an immune response that does not rely on the production of antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.

In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Professional phagocytes include macrophages, neutrophils, and dendritic cells (DCs).

The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates. The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants, fungi, prokaryotes, and invertebrates.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.

An alveolar macrophage, pulmonary macrophage, is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.

Macrophage receptor with collagenous structure (MARCO) is a protein that in humans is encoded by the MARCO gene. MARCO is a class A scavenger receptor that is found on particular subsets of macrophages. Scavenger receptors are pattern recognition receptors (PRRs) found most commonly on immune cells. Their defining feature is that they bind to polyanions and modified forms of a type of cholesterol called low-density lipoprotein (LDL). MARCO is able to bind and phagocytose these ligands and pathogen-associated molecular patterns (PAMPs), leading to the clearance of pathogens and cell signaling events that lead to inflammation. As part of the innate immune system, MARCO clears, or scavenges, pathogens, which leads to inflammatory responses. The scavenger receptor cysteine-rich (SRCR) domain at the end of the extracellular side of MARCO binds ligands to activate the subsequent immune responses. MARCO expression on macrophages has been associated with tumor development and also with Alzheimer's disease, via decreased responses of cells when ligands bind to MARCO.
A macrophage-activating factor (MAF) is a lymphokine or other receptor based signal that primes macrophages towards cytotoxicity to tumors, cytokine secretion, or clearance of pathogens. Similar molecules may cause development of an inhibitory, regulatory phenotype. A MAF can also alter the ability of macrophages to present MHC I antigen, participate in Th responses, and/or affect other immune responses.
An inflammatory cytokine or proinflammatory cytokine is a type of signaling molecule that is secreted from immune cells like helper T cells (Th) and macrophages, and certain other cell types that promote inflammation. They include interleukin-1 (IL-1), IL-6, IL-12, and IL-18, tumor necrosis factor alpha (TNF-α), interferon gamma (IFNγ), and granulocyte-macrophage colony stimulating factor (GM-CSF) and play an important role in mediating the innate immune response. Inflammatory cytokines are predominantly produced by and involved in the upregulation of inflammatory reactions.
Adipose tissue macrophages (ATMs) comprise resident macrophages present in adipose tissue. Besides adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells that includes pre-adipocytes, fibroblasts, vascular endothelial cells, and a large variety of immune cells. The latter ones are composed of mast cells, eosinophils, B cells, T cells and macrophages. The number of macrophages within adipose tissue differs depending on the metabolic status. As discovered by Rudolph Leibel and Anthony Ferrante et al. in 2003 at Columbia University, the percentage of macrophages within adipose tissue ranges from 10% in lean mice and humans up to 50% in obese leptin deficient mice, and up to 40% in obese humans. ATMs comprise nearly 50% of all immune cells in normal conditions, suggesting an important role in supporting normal functioning of the adipose tissue. Increased number of adipose tissue macrophages may correlate with increased production of pro-inflammatory molecules and might therefore contribute to the pathophysiological consequences of obesity, although is becoming recognized that in healthy conditions tissue-resident macrophages actively support a variety of critical physiological functions in nearly all organs and tissues, including adipose tissue.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues. The CNS is typically an immunologically privileged site because peripheral immune cells are generally blocked by the blood–brain barrier (BBB), a specialized structure composed of astrocytes and endothelial cells. However, circulating peripheral immune cells may surpass a compromised BBB and encounter neurons and glial cells expressing major histocompatibility complex molecules, perpetuating the immune response. Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier may occur.
Macrophage polarization is a process by which macrophages adopt different functional programs in response to the signals from their microenvironment. This ability is connected to their multiple roles in the organism: they are powerful effector cells of the innate immune system, but also important in removal of cellular debris, embryonic development and tissue repair.
Dermal macrophages are macrophages in the skin that facilitate skin homeostasis by mediating wound repair, hair growth, and salt balance. Their functional role in these processes is the mediator of inflammation. They can acquire an M1 or M2 phenotype to promote or suppress an inflammatory response, thereby influencing other cells' activity via the production of pro-inflammatory or anti-inflammatory cytokines. Dermal macrophages' ability to acquire pro-inflammatory properties also potentiates them in cancer defence. M1 macrophages can suppress tumour growth in the skin by their pro-inflammatory properties. However, M2 macrophages support tumour growth and invasion by the production of Th2 cytokines such as TGFβ and IL-10. Thus, the exact contribution of each phenotype to cancer defence and the skin's homeostasis is still unclear.
Immune system contribution to regeneration of tissues generally involves specific cellular components, transcription of a wide variety of genes, morphogenesis, epithelia renewal and proliferation of damaged cell types. However, current knowledge reveals more and more studies about immune system influence that cannot be omitted. As the immune system exhibits inhibitory or inflammatory functions during regeneration, the therapies are focused on either stopping these processes or control the immune cells setting in a regenerative way, suggesting that interplay between damaged tissue and immune system response must be well-balanced. Recent studies provide evidence that immune components are required not only after body injury but also in homeostasis or senescent cells replacement.




















