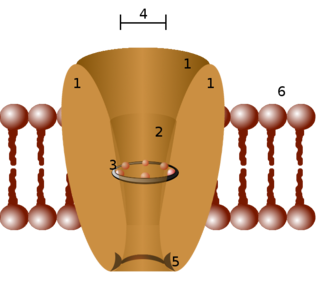
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of taste, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
TRPV6 is a membrane calcium (Ca2+) channel protein which is particularly involved in the first step in Ca2+absorption in the intestine.
TRPC is a family of transient receptor potential cation channels in animals.
TRPM is a family of transient receptor potential ion channels (M standing for wikt:melastatin). Functional TRPM channels are believed to form tetramers. The TRPM family consists of eight different channels, TRPM1–TRPM8.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.

Short transient receptor potential channel 3 (TrpC3) also known as transient receptor protein 3 (TRP-3) is a protein that in humans is encoded by the TRPC3 gene. The TRPC3/6/7 subfamily are implicated in the regulation of vascular tone, cell growth, proliferation and pathological hypertrophy. These are diacylglycerol-sensitive cation channels known to regulate intracellular calcium via activation of the phospholipase C (PLC) pathway and/or by sensing Ca2+ store depletion. Together, their role in calcium homeostasis has made them potential therapeutic targets for a variety of central and peripheral pathologies.
Short transient receptor potential channel 5 (TrpC5) also known as transient receptor protein 5 (TRP-5) is a protein that in humans is encoded by the TRPC5 gene. TrpC5 is subtype of the TRPC family of mammalian transient receptor potential ion channels.
Transient receptor potential cation channel, subfamily M, member 2, also known as TRPM2, is a protein that in humans is encoded by the TRPM2 gene.
Transient receptor potential cation channel subfamily M member 5 (TRPM5), also known as long transient receptor potential channel 5 is a protein that in humans is encoded by the TRPM5 gene.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily M member 4 (hTRPM4), also known as melastatin-4, is a protein that in humans is encoded by the TRPM4 gene.

Transient receptor potential cation channel subfamily V member 4 is an ion channel protein that in humans is encoded by the TRPV4 gene.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

Transient receptor potential cation channel subfamily M member 3 is a protein that in humans is encoded by the TRPM3 gene.

Transient receptor potential cation channel subfamily V member 5 is a calcium channel protein that in humans is encoded by the TRPV5 gene.
The transient receptor potential Ca2+ channel (TRP-CC) family (TC# 1.A.4) is a member of the voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology:
- the canonical or classic TRPs,
- the vanilloid receptor TRPs,
- the melastatin or long TRPs,
- ankyrin (whose only member is the transmembrane protein 1 [TRPA1])
- TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins,
- the polycystins
- and mucolipins.
Alexander G. Obukhov is an American researcher, who specializes in ion channels, molecular physiology, and vascular biology. Since 1986, Obukhov published research articles, with the most notable ones published in academic journals such as Nature, Journal of Biological Chemistry, EMBO Journal, Journal of Cell Biology, Proceedings of the National Academy of Sciences of the United States of America, and Neuron. Obukhov's research later evolved to feature multiple fields including neurophysiology, traumatic brain injury, pain, and atherosclerosis.

















