
Pseudomonas fluorescens is a common Gram-negative, rod-shaped bacterium. It belongs to the Pseudomonas genus; 16S rRNA analysis as well as phylogenomic analysis has placed P. fluorescens in the P. fluorescens group within the genus, to which it lends its name.

Phage therapy, viral phage therapy, or phagotherapy is the therapeutic use of bacteriophages for the treatment of pathogenic bacterial infections. This therapeutic approach emerged at the beginning of the 20th century but was progressively replaced by the use of antibiotics in most parts of the world after the Second World War. Bacteriophages, known as phages, are a form of virus that attach to bacterial cells and inject their genome into the cell. The bacteria's production of the viral genome interferes with its ability to function, halting the bacterial infection. The bacterial cell causing the infection is unable to reproduce and instead produces additional phages. Phages are very selective in the strains of bacteria they are effective against.
A slime layer in bacteria is an easily removable, unorganized layer of extracellular material that surrounds bacteria cells. Specifically, this consists mostly of exopolysaccharides, glycoproteins, and glycolipids. Therefore, the slime layer is considered as a subset of glycocalyx.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. P. aeruginosa is able to selectively inhibit various antibiotics from penetrating its outer membrane - and has high resistance to several antibiotics. According to the World Health Organization P. aeruginosa poses one of the greatest threats to humans in terms of antibiotic resistance.
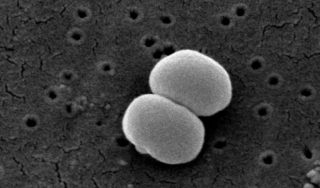
Staphylococcus epidermidis is a Gram-positive bacterium, and one of over 40 species belonging to the genus Staphylococcus. It is part of the normal human microbiota, typically the skin microbiota, and less commonly the mucosal microbiota and also found in marine sponges. It is a facultative anaerobic bacteria. Although S. epidermidis is not usually pathogenic, patients with compromised immune systems are at risk of developing infection. These infections are generally hospital-acquired. S. epidermidis is a particular concern for people with catheters or other surgical implants because it is known to form biofilms that grow on these devices. Being part of the normal skin microbiota, S. epidermidis is a frequent contaminant of specimens sent to the diagnostic laboratory.
Stenotrophomonas maltophilia is an aerobic, nonfermentative, Gram-negative bacterium. It is an uncommon bacterium and human infection is difficult to treat. Initially classified as Bacterium bookeri, then renamed Pseudomonas maltophilia, S. maltophilia was also grouped in the genus Xanthomonas before eventually becoming the type species of the genus Stenotrophomonas in 1993.
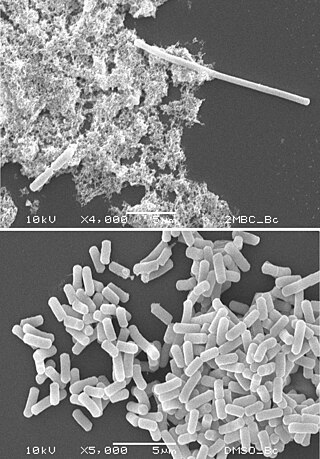
Filamentation is the anomalous growth of certain bacteria, such as Escherichia coli, in which cells continue to elongate but do not divide. The cells that result from elongation without division have multiple chromosomal copies.

An efflux pump is an active transporter in cells that moves out unwanted material. Efflux pumps are an important component in bacteria in their ability to remove antibiotics. The efflux could also be the movement of heavy metals, organic pollutants, plant-produced compounds, quorum sensing signals, bacterial metabolites and neurotransmitters. All microorganisms, with a few exceptions, have highly conserved DNA sequences in their genome that encode efflux pumps. Efflux pumps actively move substances out of a microorganism, in a process known as active efflux, which is a vital part of xenobiotic metabolism. This active efflux mechanism is responsible for various types of resistance to bacterial pathogens within bacterial species - the most concerning being antibiotic resistance because microorganisms can have adapted efflux pumps to divert toxins out of the cytoplasm and into extracellular media.

Acinetobacter baumannii is a typically short, almost round, rod-shaped (coccobacillus) Gram-negative bacterium. It is named after the bacteriologist Paul Baumann. It can be an opportunistic pathogen in humans, affecting people with compromised immune systems, and is becoming increasingly important as a hospital-derived (nosocomial) infection. While other species of the genus Acinetobacter are often found in soil samples, it is almost exclusively isolated from hospital environments. Although occasionally it has been found in environmental soil and water samples, its natural habitat is still not known.
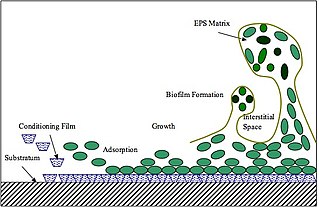
Extracellular polymeric substances (EPSs) are natural polymers of high molecular weight secreted by microorganisms into their environment. EPSs establish the functional and structural integrity of biofilms, and are considered the fundamental component that determines the physicochemical properties of a biofilm. EPS in the matrix of biofilms provides compositional support and protection of microbial communities from the harsh environments. Components of EPS can be of different classes of polysaccharides, lipids, nucleic acids, proteins, lipopolysaccharides, and minerals.
Persister cells are subpopulations of cells that resist treatment, and become antimicrobial tolerant by changing to a state of dormancy or quiescence. Persister cells in their dormancy do not divide. The tolerance shown in persister cells differs from antimicrobial resistance in that the tolerance is not inherited and is reversible. When treatment has stopped the state of dormancy can be reversed and the cells can reactivate and multiply. Most persister cells are bacterial, and there are also fungal persister cells, yeast persister cells, and cancer persister cells that show tolerance for cancer drugs.
Roberto Kolter is Professor of Microbiology, Emeritus at Harvard Medical School, an author, and past president of the American Society for Microbiology. Kolter has been a professor at Harvard Medical School since 1983 and was Co-director of Harvard's Microbial Sciences Initiative from 2003-2018. During the 35-year term of the Kolter laboratory from 1983 to 2018, more than 130 graduate student and postdoctoral trainees explored an eclectic mix of topics gravitating around the study of microbes. Kolter is a fellow of the American Association for the Advancement of Science and of the American Academy of Microbiology.
Biofilm formation occurs when free floating microorganisms attach themselves to a surface. Although there are some beneficial uses of biofilms, they are generally considered undesirable, and means of biofilm prevention have been developed. Biofilms secrete extracellular polymeric substance that provides a structural matrix and facilitates adhesion for the microorganisms; the means of prevention have thus concentrated largely on two areas: killing the microbes that form the film, or preventing the adhesion of the microbes to a surface. Because biofilms protect the bacteria, they are often more resistant to traditional antimicrobial treatments, making them a serious health risk. For example, there are more than one million cases of catheter-associated urinary tract infections (CAUTI) reported each year, many of which can be attributed to bacterial biofilms. There is much research into the prevention of biofilms.
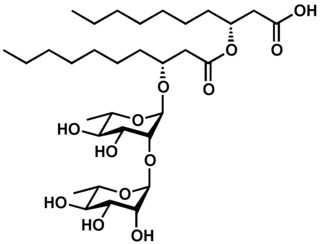
Rhamnolipids are a class of glycolipid produced by Pseudomonas aeruginosa, amongst other organisms, frequently cited as bacterial surfactants. They have a glycosyl head group, in this case a rhamnose moiety, and a 3-(hydroxyalkanoyloxy)alkanoic acid (HAA) fatty acid tail, such as 3-hydroxydecanoic acid.
Bacterial morphological plasticity refers to changes in the shape and size that bacterial cells undergo when they encounter stressful environments. Although bacteria have evolved complex molecular strategies to maintain their shape, many are able to alter their shape as a survival strategy in response to protist predators, antibiotics, the immune response, and other threats.
ESKAPE is an acronym comprising the scientific names of six highly virulent and antibiotic resistant bacterial pathogens including: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. The acronym is sometimes extended to ESKAPEE to include Escherichia coli. This group of Gram-positive and Gram-negative bacteria can evade or 'escape' commonly used antibiotics due to their increasing multi-drug resistance (MDR). As a result, throughout the world, they are the major cause of life-threatening nosocomial or hospital-acquired infections in immunocompromised and critically ill patients who are most at risk. P. aeruginosa and S. aureus are some of the most ubiquitous pathogens in biofilms found in healthcare. P. aeruginosa is a Gram-negative, rod-shaped bacterium, commonly found in the gut flora, soil, and water that can be spread directly or indirectly to patients in healthcare settings. The pathogen can also be spread in other locations through contamination, including surfaces, equipment, and hands. The opportunistic pathogen can cause hospitalized patients to have infections in the lungs, blood, urinary tract, and in other body regions after surgery. S. aureus is a Gram-positive, cocci-shaped bacterium, residing in the environment and on the skin and nose of many healthy individuals. The bacterium can cause skin and bone infections, pneumonia, and other types of potentially serious infections if it enters the body. S. aureus has also gained resistance to many antibiotic treatments, making healing difficult. Because of natural and unnatural selective pressures and factors, antibiotic resistance in bacteria usually emerges through genetic mutation or acquires antibiotic-resistant genes (ARGs) through horizontal gene transfer - a genetic exchange process by which antibiotic resistance can spread.
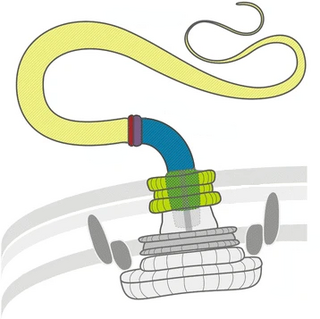
Twitching motility is a form of crawling bacterial motility used to move over surfaces. Twitching is mediated by the activity of hair-like filaments called type IV pili which extend from the cell's exterior, bind to surrounding solid substrates, and retract, pulling the cell forwards in a manner similar to the action of a grappling hook. The name twitching motility is derived from the characteristic jerky and irregular motions of individual cells when viewed under the microscope. It has been observed in many bacterial species, but is most well studied in Pseudomonas aeruginosa, Neisseria gonorrhoeae and Myxococcus xanthus. Active movement mediated by the twitching system has been shown to be an important component of the pathogenic mechanisms of several species.
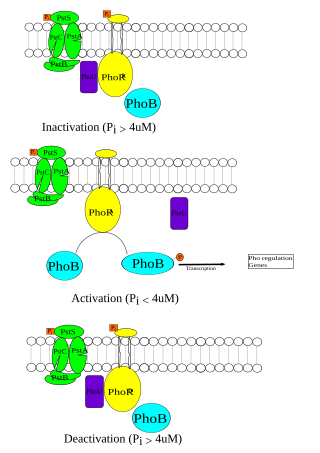
The Phosphate (Pho) regulon is a regulatory mechanism used for the conservation and management of inorganic phosphate within the cell. It was first discovered in Escherichia coli as an operating system for the bacterial strain, and was later identified in other species. The Pho system is composed of various components including extracellular enzymes and transporters that are capable of phosphate assimilation in addition to extracting inorganic phosphate from organic sources. This is an essential process since phosphate plays an important role in cellular membranes, genetic expression, and metabolism within the cell. Under low nutrient availability, the Pho regulon helps the cell survive and thrive despite a depletion of phosphate within the environment. When this occurs, phosphate starvation-inducible (psi) genes activate other proteins that aid in the transport of inorganic phosphate.
Niels Høiby is a Danish physician, professor and politician. He specialises in microbiology and was a pioneer in the study of biofilms and their role in conditions such as cystic fibrosis. He worked for many years as a department head at Denmark's largest hospital, the Rigshospitalet.
Diffusible signal factor (DSF) is found in several gram-negative bacteria and play a role in the formation of biofilms, motility, virulence, and antibiotic resistance. Xanthomonas campestris was the first bacteria known to have DSF. The synthesis of the DSF can be seen in rpfF and rpfB enzymes. An understanding of the DSF signaling mechanism could lead to further disease control.























