Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer (sacculus) that surrounds the bacterial cytoplasmic membrane. The sugar component consists of alternating residues of β-(1,4) linked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). Attached to the N-acetylmuramic acid is an oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Peptidoglycan hydrolysis and synthesis are two processes that must occur in order for cells to grow and multiply, a technique carried out in three stages: clipping of current material, insertion of new material, and re-crosslinking of existing material to new material.

Tumor necrosis factor is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.
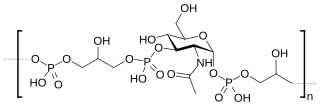
Teichoic acids are bacterial copolymers of glycerol phosphate or ribitol phosphate and carbohydrates linked via phosphodiester bonds.

Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide that are bacterial toxins. They are composed of an O-antigen, an outer core, and an inner core all joined by covalent bonds, and are found in the outer membrane of Gram-negative bacteria, such as E. coli and Salmonella. Today, the term endotoxin is often used synonymously with LPS, although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins produced by Bacillus thuringiensis.
The cell envelope comprises the inner cell membrane and the cell wall of a bacterium. In gram-negative bacteria an outer membrane is also included. This envelope is not present in the Mollicutes where the cell wall is absent.

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes, but not present in the host. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. This allows the innate immune system to recognize pathogens and thus, protect the host from infection.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

Lacticaseibacillus rhamnosus is a bacterium that originally was considered to be a subspecies of L. casei, but genetic research found it to be a separate species in the L. casei clade, which also includes L. paracasei and L. zeae. It is a short Gram-positive homofermentative facultative anaerobic non-spore-forming rod that often appears in chains. Some strains of L. rhamnosus bacteria are being used as probiotics, and are particularly useful in treating infections of the female urogenital tract, most particularly very difficult to treat cases of bacterial vaginosis. The species Lacticaseibacillus rhamnosus and Limosilactobacillus reuteri are commonly found in the healthy female genito-urinary tract and are helpful to regain control of dysbiotic bacterial overgrowth during an active infection. L. rhamnosus sometimes is used in dairy products such as fermented milk and as non-starter-lactic acid bacterium (NSLAB) in long-ripened cheese. While frequently considered a beneficial organism, L. rhamnosus may not be as beneficial to certain subsets of the population; in rare circumstances, especially those primarily involving weakened immune system or infants, it may cause endocarditis. Despite the rare infections caused by L. rhamnosus, the species is included in the list of bacterial species with qualified presumed safety (QPS) status of the European Food Safety Agency.

CD14 is a human protein made mostly by macrophages as part of the innate immune system. It helps to detect bacteria in the body by binding lipopolysaccharide (LPS), a pathogen-associated molecular pattern (PAMP).
Virulence factors are cellular structures, molecules and regulatory systems that enable microbial pathogens to achieve the following:

Interleukin-26 (IL-26) is a protein that in humans is encoded by the IL26 gene.

Toll-like receptor 2 also known as TLR2 is a protein that in humans is encoded by the TLR2 gene. TLR2 has also been designated as CD282. TLR2 is one of the toll-like receptors and plays a role in the immune system. TLR2 is a membrane protein, a receptor, which is expressed on the surface of certain cells and recognizes foreign substances and passes on appropriate signals to the cells of the immune system.
The formyl peptide receptors (FPR) belong to a class of G protein-coupled receptors involved in chemotaxis. In humans, there are three formyl peptide receptor isoforms, each encoded by a separate gene that are named FPR1, FPR2, and FPR3. These receptors were originally identified by their ability to bind N-formyl peptides such as N-formylmethionine produced by the degradation of either bacterial or host cells. Hence formyl peptide receptors are involved in mediating immune cell response to infection. These receptors may also act to suppress the immune system under certain conditions. The close phylogenetic relation of signaling in chemotaxis and olfaction was recently proved by detection formyl peptide receptor like proteins as a distinct family of vomeronasal organ chemosensors in mice.

Toll-like receptor 5, also known as TLR5, is a protein which in humans is encoded by the TLR5 gene. It is a member of the toll-like receptor (TLR) family. TLR5 is known to recognize bacterial flagellin from invading mobile bacteria. It has been shown to be involved in the onset of many diseases, which includes Inflammatory bowel disease. Recent studies have also shown that malfunctioning of TLR5 is likely related to rheumatoid arthritis, osteoclastogenesis, and bone loss. Abnormal TLR5 functioning is related to the onset of gastric, cervical, endometrial and ovarian cancers.

Toll-like receptor 4 is a protein that in humans is encoded by the TLR4 gene. TLR4 is a transmembrane protein, member of the toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. Its activation leads to an intracellular signaling pathway NF-κB and inflammatory cytokine production which is responsible for activating the innate immune system.

Toll-like receptor 6 is a protein that in humans is encoded by the TLR6 gene. TLR6 is a transmembrane protein, member of toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. TLR6 acts in a heterodimer form with toll-like receptor 2 (TLR2). Its ligands include multiple diacyl lipopeptides derived from gram-positive bacteria and mycoplasma and several fungal cell wall saccharides. After dimerizing with TLR2, the NF-κB intracellular signalling pathway is activated, leading to a pro-inflammatory cytokine production and activation of innate immune response. TLR6 has also been designated as CD286.

Programmed death-ligand 1 (PD-L1) also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a protein that in humans is encoded by the CD274 gene.
Serum amyloid A1 (SAA1) is a protein that in humans is encoded by the SAA1 gene. SAA1 is a major acute-phase protein mainly produced by hepatocytes in response to infection, tissue injury and malignancy. When released into blood circulation, SAA1 is present as an apolipoprotein associated with high-density lipoprotein (HDL). SAA1 is a major precursor of amyloid A (AA), the deposit of which leads to inflammatory amyloidosis.

Roman Dziarski is a Polish-born American immunologist and microbiologist. He is best known for his research on innate immunity and bacterial peptidoglycan, for discovering the family of human peptidoglycan recognition proteins, which comprises PGLYRP1, PGLYRP2, PGLYRP3, and PGLYRP4, and for defining the functions of these proteins.















