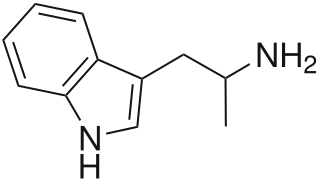
α-Methyltryptamine is a psychedelic, stimulant, and entactogen drug of the tryptamine family. It was originally developed as an antidepressant at Upjohn in the 1960s, and was used briefly as an antidepressant in the Soviet Union under the brand name Indopan or Indopane before being discontinued.
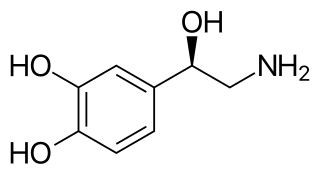
Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems.

(–)-Benzofuranylpropylaminopentane is an experimental drug related to selegiline which acts as a monoaminergic activity enhancer (MAE). It is orally active in animals.

Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the TAAR1 gene. TAAR1 is an intracellular amine-activated Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells, astrocytes, and in the intracellular milieu within the presynaptic plasma membrane of monoamine neurons in the central nervous system (CNS). TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski et al. and Bunzow et al. TAAR1 is one of six functional human trace amine-associated receptors, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmune system function through different mechanisms.

5,7-Dihydroxytryptamine (5,7-DHT) is a monoaminergic neurotoxin used in scientific research to decrease concentrations of serotonin in the brain. The mechanism behind this effect is not well understood, but it is speculated to selectively destroy serotonergic neurons, in a manner similar to the dopaminergic neurotoxicity of 6-hydroxydopamine (6-OHDA). What is known is that this compound is in fact not selective in depleting serotonin content, but also depletes norepinephrine. To selectively deplete serotonin stores, it is commonly administered in conjunction with desmethylimipramine (desipramine), which inhibits the norepinephrine transporter.

Pheniprazine, formerly sold under the brand names Catron and Cavodil, is an irreversible and non-selective monoamine oxidase inhibitor (MAOI) of the hydrazine group that was used as an antidepressant to treat depression in the 1960s. It was also used in the treatment of angina pectoris and schizophrenia. Pheniprazine has been largely discontinued due to toxicity concerns such as jaundice, amblyopia, and optic neuritis.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.
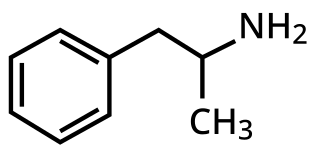
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.

2-Aminotetralin (2-AT), also known as 1,2,3,4-tetrahydronaphthalen-2-amine (THN), is a stimulant drug with a chemical structure consisting of a tetralin group combined with an amine.

3-Fluoroamphetamine is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin. It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.

Amiflamine (FLA-336) is a reversible inhibitor of monoamine oxidase A (MAO-A), thereby being a RIMA, and, to a lesser extent, semicarbazide-sensitive amine oxidase (SSAO), as well as a serotonin releasing agent (SRA). It is a derivative of the phenethylamine and amphetamine chemical classes. The (+)-enantiomer is the active stereoisomer.
EXP-561 is an investigational drug that acts as an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. It was developed in the 1960s by Du Pont and was suggested as a potential antidepressant but failed in trials and was never marketed.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.

Monoaminergic activity enhancers (MAE), also known as catecholaminergic/serotonergic activity enhancers (CAE/SAE), are a class of compounds that enhance the action potential-evoked release of monoamine neurotransmitters in the nervous system. MAEs are distinct from monoamine releasing agents (MRAs) like amphetamine and fenfluramine in that they do not induce the release of monoamines from synaptic vesicles but rather potentiate only nerve impulse propagation-mediated monoamine release. That is, MAEs increase the amounts of monoamine neurotransmitters released by neurons per electrical impulse.

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine. Examples of monoamine neurotoxins include the serotonergic neurotoxins para-chloroamphetamine (PCA), methylenedioxymethamphetamine (MDMA), and 5,7-dihydroxytryptamine (5,7-DHT); the dopaminergic neurotoxins oxidopamine (6-hydroxydopamine), MPTP, and methamphetamine; and the noradrenergic neurotoxins oxidopamine and DSP-4. Dopaminergic neurotoxins can induce a Parkinson's disease-like condition in animals and humans. Serotonergic neurotoxins have been associated with cognitive and memory deficits and psychiatric changes.



















