
Pergolide, sold under the brand name Permax and Prascend (veterinary) among others, is an ergoline-based dopamine receptor agonist used in some countries for the treatment of Parkinson's disease. Parkinson's disease is associated with reduced dopamine activity in the substantia nigra of the brain. Pergolide acts on many of the same receptors as dopamine to increase receptor activity.

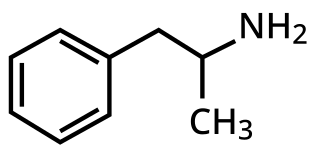
3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
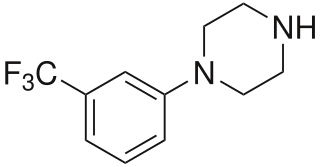
3-Trifluoromethylphenylpiperazine (TFMPP) is a recreational drug of the phenylpiperazine chemical class and is a substituted piperazine. Usually in combination with benzylpiperazine (BZP) and other analogues, it is sold as an alternative to the illicit drug MDMA ("Ecstasy").

5-(2-Aminopropyl)-2,3-dihydro-1H-indene (5-APDI), also known as indanylaminopropane (IAP), IAP (psychedelic), 2-API(2-aminopropylindane), indanametamine, and, incorrectly, as indanylamphetamine, is an entactogen and psychedelic drug of the amphetamine family. It has been sold by online vendors through the Internet and has been encountered as a designer drug since 2003, but its popularity and availability has diminished in recent years.
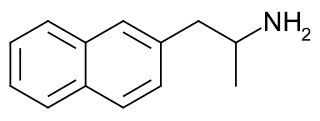
Naphthylaminopropane (PAL-287) is an experimental drug under investigation as of 2007 for the treatment of alcohol and stimulant addiction.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT).
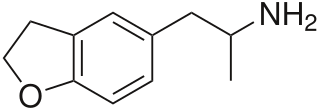
5-(2-Aminopropyl)-2,3-dihydrobenzofuran is a putative entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 3-position oxygen from the 3,4-methylenedioxy ring has been replaced by a methylene bridge. 6-APDB is an analogue of 5-APDB where the 4-position oxygen has been replaced by a methylene bridge instead. 5-APDB was developed by a team led by David E. Nichols at Purdue University as part of their research into non-neurotoxic analogues of MDMA.
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.
A serotonin–norepinephrine–dopamine releasing agent (SNDRA), also known as a triple releasing agent (TRA), is a type of drug which induces the release of serotonin, norepinephrine/epinephrine, and dopamine in the brain and body. SNDRAs produce euphoriant, entactogen, and psychostimulant effects, and are almost exclusively encountered as recreational drugs.
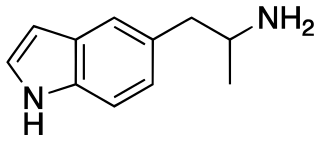
5-(2-Aminopropyl)indole is an indole and phenethylamine derivative with empathogenic effects. Its preparation was first reported by Albert Hofmann in 1962. It is a designer drug that has been openly sold as a recreational drug by online vendors since 2011.
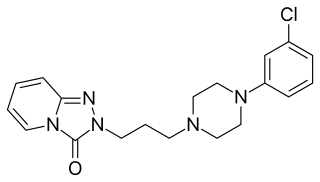
Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

6-(2-Aminopropyl)-2,3-dihydrobenzofuran is a stimulant and entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 4-position oxygen from the 3,4-methylenedioxy ring has been replaced with a methylene bridge. 5-APDB (3-Desoxy-MDA) is an analogue of 6-APDB where the 3-position oxygen has been replaced with a methylene instead. 6-APDB, along with 5-APDB, was first synthesized by David E. Nichols in the early 1990s while investigating non-neurotoxic MDMA analogues.
6-APB is an empathogenic psychoactive compound of the substituted benzofuran and substituted phenethylamine classes. 6-APB and other compounds are sometimes informally called "Benzofury" in newspaper reports. It is similar in structure to MDA, but differs in that the 3,4-methylenedioxyphenyl ring system has been replaced with a benzofuran ring. 6-APB is also the unsaturated benzofuran derivative of 6-APDB. It may appear as a tan grainy powder. While the drug never became particularly popular, it briefly entered the rave and underground clubbing scene in the UK before its sale and import were banned. It falls under the category of research chemicals, sometimes called "legal highs." Because 6-APB and other substituted benzofurans have not been explicitly outlawed in some countries, they are often technically legal, contributing to their popularity.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.
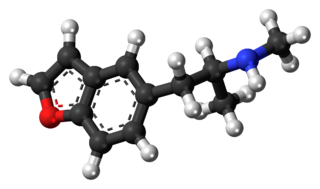
5-MAPB is an entactogenic designer drug similar to MDMA in its structure and effects.

5-MAPDB (1-(2,3-dihydrobenzofuran-5-yl)-N-methylpropan-2-amine) is a chemical compound which acts as an entactogenic drug. It is structurally related to drugs like 5-APDB and 5-MAPB, which have similar effects to MDMA and have been used as recreational drugs. 5-MAPDB has been studied to determine its pharmacological activity, and was found to be a relatively selective serotonin releaser, though with weaker actions as a releaser of other monoamines and 5-HT2 receptor family agonist, similar to older compounds such as 5-APDB.
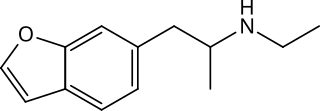
6-EAPB is a potentially psychedelic and potentially entactogenic drug of the benzofuran class; it is structurally related to 6-APB and MDMA.

The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached, and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name Benzofury.

N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.


















