
Empathogens or entactogens are a class of psychoactive drugs that induce the production of experiences of emotional communion, oneness, relatedness, emotional openness—that is, empathy or sympathy—as particularly observed and reported for experiences with 3,4-methylenedioxymethamphetamine (MDMA). This class of drug is distinguished from the classes of hallucinogen or psychedelic, and amphetamine or stimulants. Major members of this class include MDMA, MDA, MDEA, MDOH, MBDB, 5-APB, 5-MAPB, 6-APB, 6-MAPB, methylone, mephedrone, GHB, αMT, and αET, MDAI among others. Most entactogens are phenethylamines and amphetamines, although several, such as αMT and αET, are tryptamines. When referring to MDMA and its counterparts, the term MDxx is often used. Entactogens are sometimes incorrectly referred to as hallucinogens or stimulants, although many entactogens such as ecstasy exhibit psychedelic or stimulant properties as well.
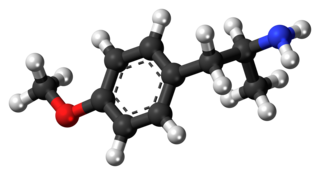
para-Methoxyamphetamine (PMA), also known as 4-methoxyamphetamine (4-MA), is a designer drug of the amphetamine class with serotonergic effects. Unlike other similar drugs of this family, PMA does not produce stimulant, euphoriant, or entactogen effects, and behaves more like an antidepressant in comparison, though it does have some psychedelic properties.

MBDB, also known as N-methyl-1,3-benzodioxolylbutanamine or as 3,4-methylenedioxy-N-methyl-α-ethylphenylethylamine, is an entactogen of the phenethylamine, amphetamine, and phenylisobutylamine families related to MDMA. It is known by the street names "Eden" and "Methyl-J".

4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

3,4-Methylenedioxyphentermine (MDPH) is a lesser-known drug of the amphetamine family. MDPH was first synthesized by Alexander Shulgin. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDPH.
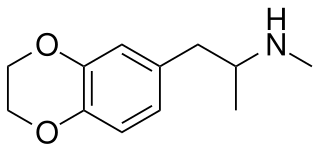
3,4-Ethylenedioxy-N-methylamphetamine (EDMA) is an entactogen drug of the methamphetamine class. It is an analogue of MDMA where the methylenedioxy ring has been replaced by an ethylenedioxy ring. EDMA was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage is listed as 150–250 mg, and the duration listed as 3–5 hours. According to Shulgin, EDMA produces a bare threshold consisting of paresthesia, nystagmus, and hypnogogic imagery, with few to no other effects.

5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.
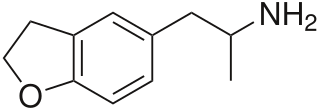
5-(2-Aminopropyl)-2,3-dihydrobenzofuran is a putative entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 3-position oxygen from the 3,4-methylenedioxy ring has been replaced by a methylene bridge. 6-APDB is an analogue of 5-APDB where the 4-position oxygen has been replaced by a methylene bridge instead. 5-APDB was developed by a team led by David E. Nichols at Purdue University as part of their research into non-neurotoxic analogues of MDMA.
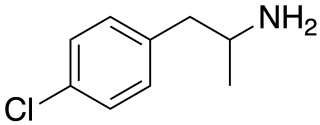
para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.

MDAI, also known as 5,6-methylenedioxy-2-aminoindane, is an entactogen drug of the 2-aminoindane group which is related to MDMA and produces similar subjective effects.
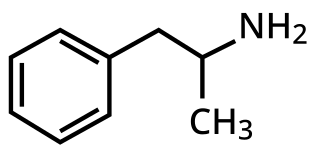
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; monoamine releasing agents can induce the release of one or more of these neurotransmitters.
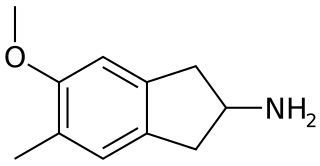
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug of the 2-aminoindane group developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a less neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogenic effects in humans. It has been sold as a designer drug and research chemical online since 2010.

5,6-Methylenedioxy-N-methyl-2-aminoindane (MDMAI), is a drug of the 2-aminoindane group developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in animals and a putative entactogen in humans.
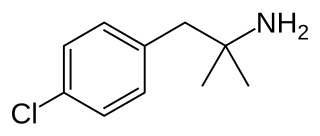
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons, including dopamine and norepinephrine neurons.
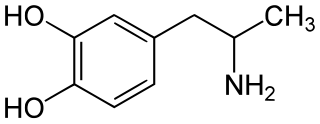
α-Methyldopamine (α-Me-DA), also known as 3,4-dihydroxyamphetamine or as catecholamphetamine, is a research chemical of the catecholamine and amphetamine families. It is a monoamine releasing agent and a metabolite of MDMA and MDA. The bis-glutathionyl metabolite of α-methyldopamine is slightly neurotoxic when directly injected into the brain's ventricles.
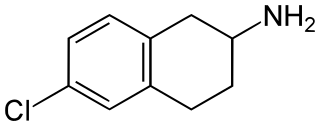
6-Chloro-2-aminotetralin (6-CAT) is a drug which acts as a selective serotonin releasing agent (SSRA) and is a putative entactogen in humans. It is a rigid analogue of para-chloroamphetamine (PCA).
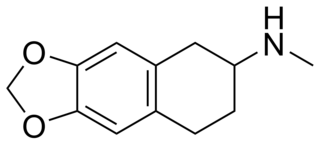
MDMAT (6,7-methylenedioxy-N-methyl-2-aminotetralin) is a selective serotonin releasing agent (SSRA) and entactogen drug. It is the N-methylated derivative of MDAT, similarly to the relationship of MDMA to MDA. It has been theorized to have less long-term neurotoxicity and less hallucinogenic effects than other MDxx derivatives, but no formal scientific research has been conducted specifically on MDMAT.

6-Methyl-3,4-methylenedioxyamphetamine (6-Methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class. It was first synthesized in the late 1990s by a team including David E. Nichols at Purdue University while investigating derivatives of 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxy-N-methylamphetamine (MDMA).
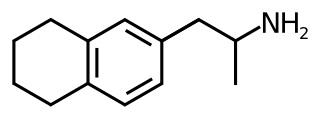
6-(2-Aminopropyl)tetralin (6-APT), also sometimes called tetralinylaminopropane (TAP), is a drug of the amphetamine class which acts as a selective serotonin releasing agent (SSRA). It has IC50 values of 121 nM, 6,436 nM, and 3,371 nM for inhibiting the reuptake of serotonin, dopamine, and norepinephrine, respectively. Though it possesses an appreciable in vitro profile, in animal drug discrimination studies it was not found to substitute for MMAI or amphetamine and to only partially substitute for MBDB. This parallels Alexander Shulgin's finding that EDMA (the 1,4-benzodioxine analogue of 6-APT) is inactive, and appears to indicate that the pharmacokinetics of both EDMA and 6-APT may not be favorable.

The Borax combo, also known by the informal brand names Blue Bliss and Pink Star, is a combination recreational and designer drug described as an MDMA-like entactogen.




















