
Verapamil, sold under various trade names, is a calcium channel blocker medication used for the treatment of high blood pressure, angina, and supraventricular tachycardia. It may also be used for the prevention of migraines and cluster headaches. It is given by mouth or by injection into a vein.
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a class of drugs that are used to suppress abnormally fast rhythms (tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular tachycardia.

Quinidine is a class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of antimalarial agent quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval. As of 2019, its IV formulation is no longer being manufactured for use in the United States.

Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of cardiac dysrhythmias. This includes ventricular tachycardia, ventricular fibrillation, and wide complex tachycardia, atrial fibrillation, and paroxysmal supraventricular tachycardia. Evidence in cardiac arrest, however, is poor. It can be given by mouth, intravenously, or intraosseously. When used by mouth, it can take a few weeks for effects to begin.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or given by injection into a vein.

Nadolol, sold under the brand name Corgard among others, is a medication used to treat high blood pressure, heart pain, atrial fibrillation, and some inherited arrhythmic syndromes. It has also been used to prevent migraine headaches and complications of cirrhosis. It is taken orally.

Procainamide (PCA) is a medication of the antiarrhythmic class used for the treatment of cardiac arrhythmias. It is a sodium channel blocker of cardiomyocytes; thus it is classified by the Vaughan Williams classification system as class Ia. In addition to blocking the INa current, it inhibits the IKr rectifier K+ current. Procainamide is also known to induce a voltage-dependent open channel block on the batrachotoxin (BTX)-activated sodium channels in cardiomyocytes.

Azimilide is a class ΙΙΙ antiarrhythmic drug. The agents from this heterogeneous group have an effect on the repolarization, they prolong the duration of the action potential and the refractory period. Also they slow down the spontaneous discharge frequency of automatic pacemakers by depressing the slope of diastolic depolarization. They shift the threshold towards zero or hyperpolarize the membrane potential. Although each agent has its own properties and will have thus a different function.
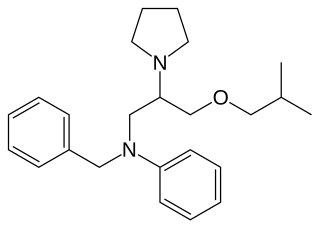
Bepridil is an diamine calcium channel blocker once used to treat angina pectoris. It is no longer sold in the United States.

Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic medication developed by Sanofi-Aventis. It was approved by the US Food and Drug Administration (FDA) in July 2009. Besides being indicated in arrhythmias, it was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm. It is a class III antiarrhythmic drug. The FDA label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in people with moderate to severe congestive heart failure.
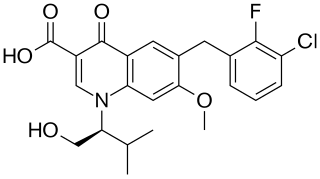
Elvitegravir (EVG) is an integrase inhibitor used to treat HIV infection. It was developed by the pharmaceutical company Gilead Sciences, which licensed EVG from Japan Tobacco in March 2008. The drug gained approval by the U.S. Food and Drug Administration on August 27, 2012, for use in adult patients starting HIV treatment for the first time as part of the fixed dose combination known as Stribild. On September 24, 2014, the FDA approved Elvitegravir as a single pill formulation under the trade name Vitekta. On November 5, 2015, the FDA approved the drug for use in patients affected with HIV-1 as a part of a second fixed dose combination pill known as Genvoya.

Cobicistat, sold under the brand name Tybost, is a medication for use in the treatment of human immunodeficiency virus infection (HIV/AIDS). Its major mechanism of action is through the inhibition of human CYP3A proteins.

F15845 is a cardiac drug proposed to have beneficial effects for the treatment of angina pectoris, arrhythmias and ischemia by inhibiting the persistent sodium current. The drug, currently in phase II of clinical trials, targets the persistent sodium current with selectivity and produces minimal adverse effects in current experimental studies.
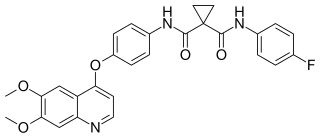
Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is an anti-cancer medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.

Budiodarone (ATI-2042) is an antiarrhythmic agent and chemical analog of amiodarone that is currently being studied in clinical trials. Amiodarone is considered the most effective antiarrhythmic drug available, but its adverse side effects, including hepatic, pulmonary and thyroid toxicity as well as multiple drug interactions, are discouraging its use. Budiodarone only differs in structure from amiodarone through the presence of a sec-butyl acetate side chain at position 2 of the benzofuran moiety. This side chain allows for budiodarone to have a shorter half-life in the body than amiodarone which allows it to have a faster onset of action and metabolism while still maintaining similar electrophysiological activity. The faster metabolism of budiodarone allows for fewer adverse side effects than amiodarone principally due to decreased levels of toxicity in the body.

AZD1305 is an experimental drug candidate that is under investigation for the management and reversal of cardiac arrhythmias, specifically atrial fibrillation and flutter. In vitro studies have shown that this combined-ion channel blocker inhibits rapidly the activating delayed-rectifier potassium current (IKr), L-type calcium current, and inward sodium current (INa).
QT prolongation is a measure of delayed ventricular repolarisation, which means the heart muscle takes longer than normal to recharge between beats. It is an electrical disturbance which can be seen on an electrocardiogram (ECG). Excessive QT prolongation can trigger tachycardias such as torsades de pointes (TdP). QT prolongation is an established side effect of antiarrhythmics, but can also be caused by a wide range of non-cardiac medicines, including antibiotics, antidepressants, antihistamines, opioids, and complementary medicines. On an ECG, the QT interval represents the summation of action potentials in cardiac muscle cells, which can be caused by an increase in inward current through sodium or calcium channels, or a decrease in outward current through potassium channels. By binding to and inhibiting the “rapid” delayed rectifier potassium current protein, certain drugs are able to decrease the outward flow of potassium ions and extend the length of phase 3 myocardial repolarization, resulting in QT prolongation.

Cobimetinib, sold under the brand name Cotellic, is an anti-cancer medication used to treat melanoma and histiocytic neoplasms. Cobimetinib is a MEK inhibitor. Cobimetinib is marketed by Genentech.
Pralsetinib, sold under the brand name Gavreto, is a medication approved for RET mutation-positive medullary thyroid cancer (MTC) and RET fusion-positive differentiated thyroid cancer (DTC) refractory to radioactive iodine (RAI) therapy. Pralsetinib is a tyrosine kinase inhibitor. It is taken by mouth.

















