Virology
| Hepatitis delta virus | |
|---|---|
 | |
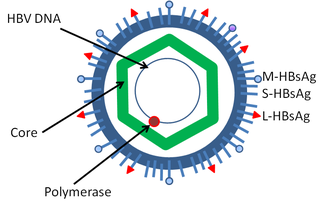
| Schematic representation of the Hepatitis delta virus virion | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Ribozyviria |
| Family: | Kolmioviridae |
| Genus: | Deltavirus |
| Species [10] | |
| |
Structure and genome
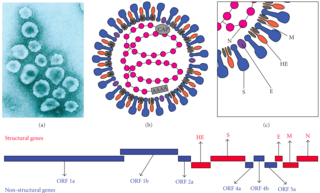
The hepatitis delta viruses, or HDV, are eight species of negative-sense single-stranded RNA viruses (or virus-like particles) classified together as the genus Deltavirus, within the realm Ribozyviria . [11] The HDV virion is a small, spherical, enveloped particle with a 36 nm diameter; its viral envelope contains host phospholipids, as well as three proteins taken from the hepatitis B virus—the large, medium, and small hepatitis B surface antigens. This assembly surrounds an inner ribonucleoprotein (RNP) particle, which contains the genome surrounded by about 200 molecules of hepatitis D antigen (HDAg) for each genome. The central region of HDAg has been shown to bind RNA. [12] Several interactions are also mediated by a coiled-coil region at the N terminus of HDAg. [13] [14]
The HDV genome is negative sense, single-stranded, closed circular RNA; with a genome of approximately 1700 nucleotides, HDV is the smallest "virus" known to infect animals. It has been proposed that HDV may have originated from a class of plant pathogens called viroids, which are much smaller than viruses. [15] [16] Its genome is unique among animal viruses because of its high GC nucleotide content. Its nucleotide sequence is about 70% self-complementary, allowing the genome to form a partially double-stranded, rod-like RNA structure. [17] HDV strains are highly divergent; fusions of different strains exist and sequences had been deposited in public databases employing different start sites for the circular viral RNA involved. This had resulted in something of chaos with respect to molecular classification of this virus, a situation which has been resolved recently with the adoption of a proposed reference genome and a uniform classification system. [18]
Life cycle
Like hepatitis B, HDV gains entry into liver cells via the sodium taurocholate cotransporting polypeptide (NTCP) [19] bile transporter. HDV recognizes its receptor via the N-terminal domain of the large hepatitis B surface antigen, HBsAg. [20] Mapping by mutagenesis of this domain has shown that amino acid residues 9–15 make up the receptor-binding site. [21] After entering the hepatocyte, the virus is uncoated and the nucleocapsid translocated to the nucleus due to a signal in HDAg [22] Since the HDV genome does not code for an RNA polymerase to replicate the virus' genome, the virus makes use of the host cellular RNA polymerases. Initially thought to use just RNA polymerase II, [23] [24] now RNA polymerases I and III have also been shown to be involved in HDV replication. [25] Normally RNA polymerase II utilizes DNA as a template and produces mRNA. Consequently, if HDV indeed utilizes RNA polymerase II during replication, it would be the only known animal pathogen capable of using a DNA-dependent polymerase as an RNA-dependent polymerase.[ citation needed ]
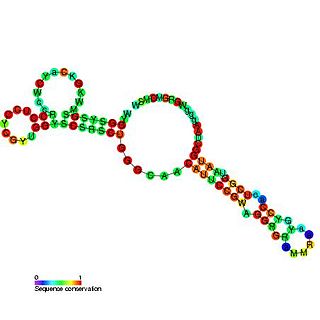
The RNA polymerases treat the RNA genome as double-stranded DNA due to the folded rod-like structure it is in. Three forms of RNA are made; circular genomic RNA, circular complementary antigenomic RNA, and a linear polyadenylated antigenomic RNA, which is the mRNA containing the open reading frame for the HDAg. Synthesis of antigenomic RNA occurs in the nucleolus, mediated by RNA polymerase I, whereas synthesis of genomic RNA takes place in the nucleoplasm, mediated by RNA polymerase II. [26] HDV RNA is synthesized first as linear RNA that contains many copies of the genome. The genomic and antigenomic RNA contain a sequence of 85 nucleotides, the hepatitis delta virus ribozyme, that acts as a ribozyme, which self-cleaves the linear RNA into monomers. These monomers are then ligated to form circular RNA. [27] [28]
Delta antigens
| Hepatitis delta virus delta antigen | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 oligomerization domain of hepatitis delta antigen | |||||||||
| Identifiers | |||||||||
| Symbol | HDV_ag | ||||||||
| Pfam | PF01517 | ||||||||
| InterPro | IPR002506 | ||||||||
| SCOP2 | 1a92 / SCOPe / SUPFAM | ||||||||
| |||||||||
A significant difference between viroids and HDV is that, while viroids produce no proteins, HDV is known to produce one protein, namely HDAg. It comes in two forms; a 27kDa large-HDAg, and a small-HDAg of 24kDa. The N-terminals of the two forms are identical, they differ by 19 more amino acids in the C-terminal of the large HDAg. [29] Both isoforms are produced from the same reading frame which contains an UAG stop codon at codon 196, which normally produces only the small-HDAg. However, editing by cellular enzyme adenosine deaminase acting on RNA (ADAR) changes the stop codon to UGG, allowing the large-HDAg to be produced. [29] [30] Despite having 90% identical sequences, these two proteins play diverging roles during the course of an infection. HDAg-S is produced in the early stages of an infection and enters the nucleus and supports viral replication. HDAg-L, in contrast, is produced during the later stages of an infection, acts as an inhibitor of viral replication, and is required for assembly of viral particles. [31] [32] [33] Thus RNA editing by the cellular enzymes is critical to the virus' life cycle because it regulates the balance between viral replication and virion assembly.[ citation needed ]
Antigenic loop infectivity
The HDV envelope protein has three of the HBV surface proteins anchored to it. The S region of the genome is most commonly expressed and its main function is to assemble subviral particles. HDV antigen proteins combine with the viral genome to form a ribonucleoprotein (RNP) which when enveloped with the subviral particles can form viral-like particles that are almost identical to mature HDV, but they are not infectious. Researchers had concluded that the determinant of infectivity of HDV was within the N-terminal pre-S1 domain of the large protein (L). It was found to be a mediator in binding to the cellular receptor. Researchers Georges Abou Jaoudé and Camille Sureau published an article in 2005 that studied the role of the antigenic loop, found in HDV envelope proteins, in the infectivity of the virus. The antigenic loop, like the N-terminal pre-S1 domain of the large protein, is exposed at the virion surface. Jaoudé and Sureau's study provided evidence that the antigenic loop may be an important factor in HDV entry into the host cell and by mutating parts of the antigenic loop, the infectivity of HDV may be minimized. [34]