FBLN1 is the gene encoding fibulin-1, an extracellular matrix and plasma protein. [5] [6] [7]
FBLN1 is the gene encoding fibulin-1, an extracellular matrix and plasma protein. [5] [6] [7]
Fibulin-1 is a secreted glycoprotein that is found in association with extracellular matrix structures including fibronectin-containing fibers, elastin-containing fibers and basement membranes. Fibulin-1 binds to a number of extracellular matrix constituents including fibronectin, [7] nidogen-1, and the proteoglycan, versican. [7] [8] Fibulin-1 is also a blood protein capable of binding to fibrinogen. [9]
Fibulin-1 has modular domain structure and includes a series of nine epidermal growth factor-like modules followed by a fibulin-type module, a module found in all members of the fibulin gene family. [6]
The human fibulin-1 gene, FBLN1, encodes four splice variants designated fibulin-1A, B, C and D, which differ in their carboxy terminal regions. In mouse, chicken and the nematode, C. elegans , only two fibulin-1 variants are produced, fibulin-1C and fibulin-1D. [5]
FBLN1 has been shown to interact with:

Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. It is approved for marketing as a topical solution in India by Central Drugs Standard Control organization in 2020 under the brand name FIBREGA for chronic wounds. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

Nidogen-1 (NID-1), formerly known as entactin, is a protein that in humans is encoded by the NID1 gene. Both nidogen-1 and nidogen-2 are essential components of the basement membrane alongside other components such as type IV collagen, proteoglycans, laminin and fibronectin.

Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major constituents of the basement membrane, namely the basal lamina. Laminins are vital to biological activity, influencing cell differentiation, migration, and adhesion.

Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.

Thrombospondin 1, abbreviated as THBS1, is a protein that in humans is encoded by the THBS1 gene.
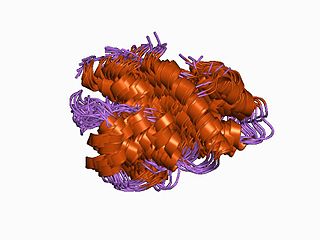
Fibulin (FY-beau-lin) is the prototypic member of a multigene family, currently with seven members. Fibulin-1 is a calcium-binding glycoprotein. In vertebrates, fibulin-1 is found in blood and extracellular matrices. In the extracellular matrix, fibulin-1 associates with basement membranes and elastic fibers. The association with these matrix structures is mediated by its ability to interact with numerous extracellular matrix constituents including fibronectin, proteoglycans, laminins and tropoelastin. In blood, fibulin-1 binds to fibrinogen and incorporates into clots.

Matrilysin also known as matrix metalloproteinase-7 (MMP-7), pump-1 protease (PUMP-1), or uterine metalloproteinase is an enzyme in humans that is encoded by the MMP7 gene. The enzyme has also been known as matrin, putative metalloproteinase-1, matrix metalloproteinase pump 1, PUMP-1 proteinase, PUMP, metalloproteinase pump-1, putative metalloproteinase, MMP). Human MMP-7 has a molecular weight around 30 kDa.

Tenascin C (TN-C) is a glycoprotein that in humans is encoded by the TNC gene. It is expressed in the extracellular matrix of various tissues during development, disease or injury, and in restricted neurogenic areas of the central nervous system. Tenascin-C is the founding member of the tenascin protein family. In the embryo it is made by migrating cells like the neural crest; it is also abundant in developing tendons, bone and cartilage.
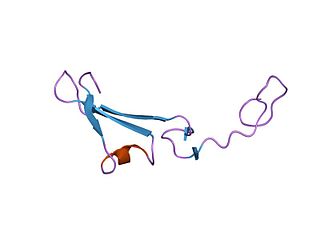
The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.

Laminin subunit alpha-5 is a protein that in humans is encoded by the LAMA5 gene.

Laminin subunit alpha-1 is a protein that in humans is encoded by the LAMA1 gene.

Laminin subunit alpha-2 is a protein that in humans is encoded by the LAMA2 gene.

Collagen alpha-3(VI) chain is a protein that in humans is encoded by the COL6A3 gene. This protein is an alpha chain of type VI collagen that aids in microfibril formation. As part of type VI collagen, this protein has been implicated in Bethlem myopathy, Ullrich congenital muscular dystrophy (UCMD), and other diseases related to muscle and connective tissue.

Fibulin-5 is a protein that in humans is encoded by the FBLN5 gene.

Fibulin-2 is a protein that in humans is encoded by the FBLN2 gene.

EGF-containing fibulin-like extracellular matrix protein 1 is a protein that in humans is encoded by the EFEMP1 gene.
EGF-containing fibulin-like extracellular matrix protein 2 is a protein that in humans is encoded by the EFEMP2 gene.

Nidogen-2, also known as osteonidogen, is a basal lamina protein of the nidogen family. It was the second nidogen to be described after nidogen-1 (entactin). Both play key roles during late embryonic development. In humans it is encoded by the NID2 gene.

Collagen alpha-1(XVI) chain is a protein that in humans is encoded by the COL16A1 gene.
Fibronectin binding protein A (FnBPA) is a Staphylococcus aureus MSCRAMM cell surface-bound protein that binds to both fibronectin and fibrinogen.