Bevacizumab, sold under the brand name Avastin among others, is a monoclonal antibody medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, ovarian cancer, glioblastoma, hepatocellular carcinoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.

Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.

Vandetanib, sold under the brand name Caprelsa, is an anti-cancer medication that is used for the treatment of certain tumours of the thyroid gland. It acts as a kinase inhibitor of a number of cell receptors, mainly the vascular endothelial growth factor receptor (VEGFR), the epidermal growth factor receptor (EGFR), and the RET-tyrosine kinase. The drug was developed by AstraZeneca who later sold the rights to Sanofi in 2015.

Semaxanib is a tyrosine-kinase inhibitor drug designed by SUGEN as a cancer therapeutic. It is an experimental stage drug, not licensed for use on human patients outside clinical trials. Semaxanib is a potent and selective synthetic inhibitor of the Flk-1/KDR vascular endothelial growth factor (VEGF) receptor tyrosine kinase. It targets the VEGF pathway, and both in vivo and in vitro studies have demonstrated antiangiogenic potential.

Temsirolimus, sold under the brand name Torisel, is an intravenous drug for the treatment of renal cell carcinoma (RCC), developed by Wyeth Pharmaceuticals and approved by the U.S. Food and Drug Administration (FDA) in May 2007, and was also approved by the European Medicines Agency (EMA) in November 2007. It is a derivative and prodrug of sirolimus.

Bosutinib, sold under the brand name Bosulif, is a small molecule BCR-ABL and src tyrosine kinase inhibitor used for the treatment of chronic myelogenous leukemia.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.

Lenvatinib, sold under the brand name Lenvima among others, is an anti-cancer medication for the treatment of certain kinds of thyroid cancer and for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases.
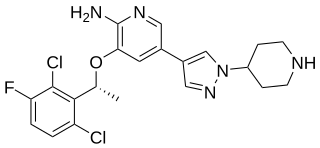
Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC). Crizotinib inhibits the c-Met/Hepatocyte growth factor receptor (HGFR) tyrosine kinase, which is involved in the oncogenesis of a number of other histological forms of malignant neoplasms. It also acts as an ALK and ROS1 inhibitor.
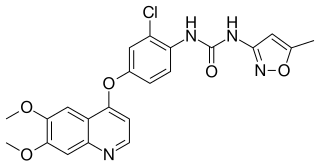
Tivozanib, sold under the brand name Fotivda, is a medication used for the treatment of advanced renal cell carcinoma. It is an oral VEGF receptor tyrosine kinase inhibitor.

Midostaurin, sold under the brand name Rydapt by Novartis, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced systemic mastocytosis. It is a semi-synthetic derivative of staurosporine, an alkaloid from the bacterium Streptomyces staurosporeus.
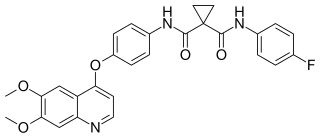
Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is an anti-cancer medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.

Ponatinib, sold under the brand name Iclusig, is a medication used for the treatment of chronic myeloid leukemia and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia. It was developed by Ariad Pharmaceuticals. It is a multi-targeted tyrosine-kinase inhibitor. Some forms of chronic myeloid leukemia, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.

Binimetinib, sold under the brand name Mektovi, is an anti-cancer medication used to treat various cancers. Binimetinib is a selective inhibitor of MEK, a central kinase in the tumor-promoting MAPK pathway. Inappropriate activation of the pathway has been shown to occur in many cancers. In June 2018 it was approved by the FDA in combination with encorafenib for the treatment of patients with unresectable or metastatic BRAF V600E or V600K mutation-positive melanoma. In October 2023, it was approved by the FDA for treatment of NSCLC with a BRAF V600E mutation in combination with encorafenib. It was developed by Array Biopharma.
Avelumab, sold under the brand name Bavencio, is a fully human monoclonal antibody medication for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma.
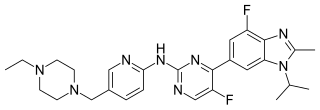
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.

Thomas E. Hutson is an American medical oncologist and cancer researcher based in Dallas, Texas. He is the director of Genitourinary Oncology Program and co-director of the Urologic Cancer Research and Treatment Center at Baylor University Medical Center. He is a Professor of Medicine at the Texas A&M Health Science Center College of Medicine and serves as a chair of Genitourinary Research for US Oncology and McKesson.
VEGFR-2 inhibitor, also known as kinase insert domain receptor(KDR) inhibitor, are tyrosine kinase receptor inhibitors that reduce angiogenesis or lymphangiogenesis, leading to anticancer activity. Generally they are small, synthesised molecules that bind competitively to the ATP-site of the tyrosine kinase domain. VEGFR-2 selective inhibitor can interrupt multiple signaling pathways involved in tumor, including proliferation, metastasis and angiogenesis.
















