Phosphofructokinase, platelet, also known as PFKP is an enzyme which in humans is encoded by the PFKP gene. [5]
Phosphofructokinase, platelet, also known as PFKP is an enzyme which in humans is encoded by the PFKP gene. [5]
The PFKP gene encodes the platelet isoform of phosphofructokinase (PFK) (ATP:D-fructose-6-phosphate-1-phosphotransferase, EC 2.7.1.11). PFK catalyzes the irreversible conversion of fructose 6-phosphate to fructose 1,6-bisphosphate and is a key regulatory enzyme in glycolysis. The PFKP gene, which maps to chromosome 10p, is also expressed in fibroblasts. See also the muscle (PFKM) and liver (PFKL) isoforms of phosphofructokinase, which map to chromosomes 12q13 and 21q22, respectively. Full tetrameric phosphofructokinase enzyme expressed in platelets can be composed of subunits P4, P3L, and P2L2. [5] [6]
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
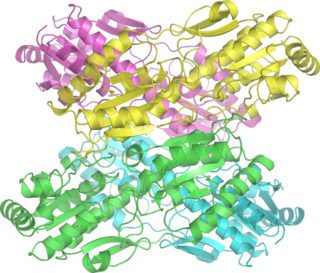
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is activated by fructose 2,6-bisphosphate. The purpose of fructose 2,6-bisphosphate is to supersede ATP inhibition, thus allowing eukaryotes to have greater sensitivity to regulation by hormones like glucagon and insulin.

Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.
Phosphofructokinase deficiency is a rare muscular metabolic disorder, with an autosomal recessive inheritance pattern.
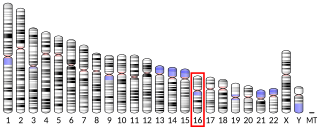
Aldolase A, also known as fructose-bisphosphate aldolase, is an enzyme that in humans is encoded by the ALDOA gene on chromosome 16.
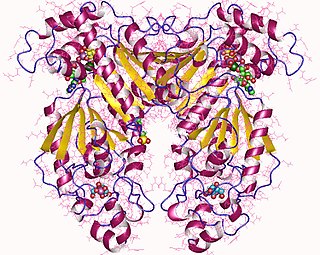
Phosphofructokinase-2 (6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of glycolysis and gluconeogenesis in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate (Fru-2,6-P2) from substrate fructose-6-phosphate. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis. Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways. Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs. This enzyme participates in fructose and mannose metabolism. The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart. In mammals, several genes often encode different isoforms, each of which differs in its tissue distribution and enzymatic activity. The family described here bears a resemblance to the ATP-driven phospho-fructokinases, however, they share little sequence similarity, although a few residues seem key to their interaction with fructose 6-phosphate.

Aldolase B also known as fructose-bisphosphate aldolase B or liver-type aldolase is one of three isoenzymes of the class I fructose 1,6-bisphosphate aldolase enzyme, and plays a key role in both glycolysis and gluconeogenesis. The generic fructose 1,6-bisphosphate aldolase enzyme catalyzes the reversible cleavage of fructose 1,6-bisphosphate (FBP) into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP) as well as the reversible cleavage of fructose 1-phosphate (F1P) into glyceraldehyde and dihydroxyacetone phosphate. In mammals, aldolase B is preferentially expressed in the liver, while aldolase A is expressed in muscle and erythrocytes and aldolase C is expressed in the brain. Slight differences in isozyme structure result in different activities for the two substrate molecules: FBP and fructose 1-phosphate. Aldolase B exhibits no preference and thus catalyzes both reactions, while aldolases A and C prefer FBP.
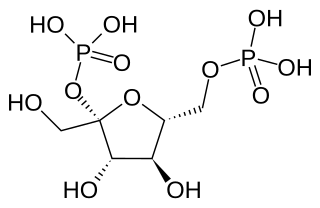
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-P2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru-2,6-P2 itself is synthesized and broken down by the bifunctional enzyme phosphofructokinase 2/fructose-2,6-bisphosphatase (PFK-2/FBPase-2).

Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.

6-phosphofructokinase, muscle type is an enzyme that in humans is encoded by the PFKM gene on chromosome 12. Three phosphofructokinase isozymes exist in humans: muscle, liver and platelet. These isozymes function as subunits of the mammalian tetramer phosphofructokinase, which catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate. Tetramer composition varies depending on tissue type. This gene encodes the muscle-type isozyme. Mutations in this gene have been associated with glycogen storage disease type VII, also known as Tarui disease. Alternatively spliced transcript variants have been described.[provided by RefSeq, Nov 2009]

PFKFB3 is a gene that encodes the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 enzyme in humans. It is one of 4 tissue-specific PFKFB isoenzymes identified currently (PFKFB1-4).
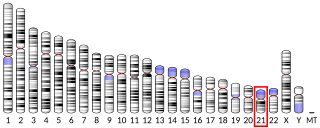
6-phosphofructokinase, liver type (PFKL) is an enzyme that in humans is encoded by the PFKL gene on chromosome 21. This gene encodes the liver (L) subunit of an enzyme that catalyzes the conversion of D-fructose 6-phosphate to D-fructose 1,6-bisphosphate, which is a key step in glucose metabolism (glycolysis). This enzyme is a tetramer that may be composed of different subunits encoded by distinct genes in different tissues. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Mar 2014]

6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 is an enzyme that in humans is encoded by the PFKFB2 gene.

Ubiquitin carboxyl-terminal hydrolase isozyme L5 is an enzyme that in humans is encoded by the UCHL5 gene.

Phosphoglucomutase-2 is an enzyme that in humans is encoded by the PGM2 gene. PGM2 is a major isozyme in red blood cells.

Aldolase C, fructose-bisphosphate, is an enzyme that, in humans, is encoded by the ALDOC gene on chromosome 17. This gene encodes a member of the class I fructose-bisphosphate aldolase gene family. Expressed specifically in the hippocampus and Purkinje cells of the brain, the encoded protein is a glycolytic enzyme that catalyzes the reversible aldol cleavage of fructose 1,6-bisphosphate and fructose-1-phosphate to dihydroxyacetone phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively.[provided by RefSeq, Jul 2008]

Tropomyosin alpha-4 chain is a protein that in humans is encoded by the TPM4 gene.

PHD finger protein 3 is a protein that in humans is encoded by the PHF3 gene.

Pyruvate kinase PKLR is an enzyme that in humans is encoded by the PKLR gene.

Forkhead box protein K1 is a transcription factor of the forkhead box family that in humans is encoded by the FOXK1 gene.

The TP53-inducible glycolysis and apoptosis regulator (TIGAR) also known as fructose-2,6-bisphosphatase TIGAR is an enzyme that in humans is encoded by the C12orf5 gene.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.