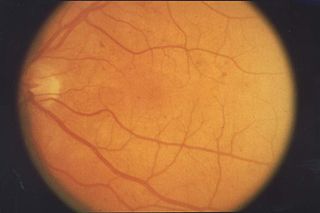
Retinopathy is any damage to the retina of the eyes, which may cause vision impairment. Retinopathy often refers to retinal vascular disease, or damage to the retina caused by abnormal blood flow. Age-related macular degeneration is technically included under the umbrella term retinopathy but is often discussed as a separate entity. Retinopathy, or retinal vascular disease, can be broadly categorized into proliferative and non-proliferative types. Frequently, retinopathy is an ocular manifestation of systemic disease as seen in diabetes or hypertension. Diabetes is the most common cause of retinopathy in the U.S. as of 2008. Diabetic retinopathy is the leading cause of blindness in working-aged people. It accounts for about 5% of blindness worldwide and is designated a priority eye disease by the World Health Organization.

Diabetic retinopathy, is a medical condition in which damage occurs to the retina due to diabetes mellitus. It is a leading cause of blindness in developed countries.

Vitrectomy is a surgery to remove some or all of the vitreous humor from the eye.
The National Eye Institute (NEI) is part of the U.S. National Institutes of Health (NIH), an agency of the U.S. Department of Health and Human Services. The mission of NEI is "to eliminate vision loss and improve quality of life through vision research." NEI consists of two major branches for research: an extramural branch that funds studies outside NIH and an intramural branch that funds research on the NIH campus in Bethesda, Maryland. Most of the NEI budget funds extramural research.

Triamcinolone acetonide, sold under the brand name Kenalog among others, is a synthetic corticosteroid medication used topically to treat various skin conditions, to relieve the discomfort of mouth sores, and by injection into joints to treat various joint conditions. It is also injected into lesions to treat inflammation in some parts of the body, particularly the skin. In nasal spray form, it is used to treat allergic rhinitis. It is used for the treatment of macular edema associated with uveitis. It is a more potent derivative of triamcinolone, and is about eight times as potent as prednisone.

Intravitreal is a route of administration of a drug, or other substance, in which the substance is delivered into the vitreous humor of the eye. "Intravitreal" literally means "inside an eye". Intravitreal injections were first introduced in 1911 when Ohm gave an injection of air into the vitreous humor to repair a detached retina. In the mid-1940s, intravitreal injections became a standard way to administer drugs to treat endophthalmitis and cytomegalovirus retinitis.
Ranibizumab, sold under the brand name Lucentis among others, is a monoclonal antibody fragment (Fab) created from the same parent mouse antibody as bevacizumab. It is an anti-angiogenic that is approved to treat the "wet" type of age-related macular degeneration, diabetic retinopathy, and macular edema due to branch retinal vein occlusion or central retinal vein occlusion.
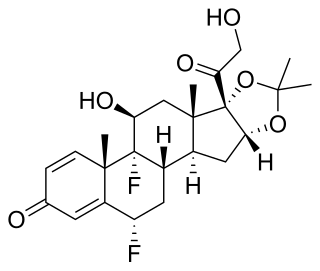
Fluocinolone acetonide is a corticosteroid primarily used in dermatology to reduce skin inflammation and relieve itching. It is a synthetic hydrocortisone derivative. The fluorine substitution at position 9 in the steroid nucleus greatly enhances its activity. It was first synthesized in 1959 in the Research Department of Syntex Laboratories S.A. Mexico City. Preparations containing it were first marketed under the name Synalar. A typical dosage strength used in dermatology is 0.01–0.025%. One such cream is sold under the brand name Flucort-N and includes the antibiotic neomycin.

Intermediate uveitis is a form of uveitis localized to the vitreous and peripheral retina. Primary sites of inflammation include the vitreous of which other such entities as pars planitis, posterior cyclitis, and hyalitis are encompassed. Intermediate uveitis may either be an isolated eye disease or associated with the development of a systemic disease such as multiple sclerosis or sarcoidosis. As such, intermediate uveitis may be the first expression of a systemic condition. Infectious causes of intermediate uveitis include Epstein–Barr virus infection, Lyme disease, HTLV-1 virus infection, cat scratch disease, and hepatitis C.

Central retinal vein occlusion, also CRVO, is when the central retinal vein becomes occluded, usually through thrombosis. The central retinal vein is the venous equivalent of the central retinal artery and both may become occluded. Since the central retinal artery and vein are the sole source of blood supply and drainage for the retina, such occlusion can lead to severe damage to the retina and blindness, due to ischemia and edema (swelling).

Macular telangiectasia is a condition of the retina, the light-sensing tissue at the back of the eye that causes gradual deterioration of central vision, interfering with tasks such as reading and driving.

Laser coagulation or laser photocoagulation surgery is used to treat a number of eye diseases and has become widely used in recent decades. During the procedure, a laser is used to finely cauterize ocular blood vessels to attempt to bring about various therapeutic benefits.

Branch retinal vein occlusion is a common retinal vascular disease of the elderly. It is caused by the occlusion of one of the branches of central retinal vein.

Vitreomacular adhesion (VMA) is a human medical condition where the vitreous gel of the human eye adheres to the retina in an abnormally strong manner. As the eye ages, it is common for the vitreous to separate from the retina. But if this separation is not complete, i.e. there is still an adhesion, this can create pulling forces on the retina that may result in subsequent loss or distortion of vision. The adhesion in of itself is not dangerous, but the resulting pathological vitreomacular traction (VMT) can cause severe ocular damage.
Anti–vascular endothelial growth factor therapy, also known as anti-VEGF therapy or medication, is the use of medications that block vascular endothelial growth factor. This is done in the treatment of certain cancers and in age-related macular degeneration. They can involve monoclonal antibodies such as bevacizumab, antibody derivatives such as ranibizumab (Lucentis), or orally-available small molecules that inhibit the tyrosine kinases stimulated by VEGF: sunitinib, sorafenib, axitinib, and pazopanib.
Cochrane Eyes and Vision (CEV) is a collaboration of researchers and healthcare professionals who prepare systematic reviews to study interventions pertaining to the treatment of eye disease and visual impairment. Though many of the systematic reviews focus on common eye diseases, reviews have been prepared for varied eye topics, including screening prevention and rarer eye diseases.

Faricimab, sold under the brand name Vabysmo, is a monoclonal antibody used for the treatment of neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). Faricimab is the first bispecific monoclonal antibody to target both vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang-2). By targeting these pathways, faricimab stabilizes blood vessels in the retina. It is given by intravitreal injection by an ophthalmologist.

Intravitreal injection is the method of administration of drugs into the eye by injection with a fine needle. The medication will be directly applied into the vitreous humor. It is used to treat various eye diseases, such as age-related macular degeneration (AMD), diabetic retinopathy, and infections inside the eye such as endophthalmitis. As compared to topical administration, this method is beneficial for a more localized delivery of medications to the targeted site, as the needle can directly pass through the anatomical eye barrier and dynamic barrier. It could also minimize adverse drug effects on other body tissues via the systemic circulation, which could be a possible risk for intravenous injection of medications. Although there are risks of infections or other complications, with suitable precautions throughout the injection process, chances for these complications could be lowered.
Sickle cell retinopathy can be defined as retinal changes due to blood vessel damage in the eye of a person with a background of sickle cell disease. It can likely progress to loss of vision in late stages due to vitreous hemorrhage or retinal detachment. Sickle cell disease is a structural red blood cell disorder leading to consequences in multiple systems. It is characterized by chronic red blood cell destruction, vascular injury, and tissue ischemia causing damage to the brain, eyes, heart, lungs, kidneys, spleen, and musculoskeletal system.
Conbercept, sold under the commercial name Lumitin, is a novel vascular endothelial growth factor (VEGF) inhibitor used to treat neovascular age-related macular degeneration (AMD) and diabetic macular edema (DME). The anti-VEGF was approved for the treatment of neovascular AMD by the China State FDA (CFDA) in December 2013. As of December 2020, conbercept is undergoing phase III clinical trials through the U.S. Food and Drug Administration’s PANDA-1 and PANDA-2 development programs.















