Volume 31, Number 1—January 2025
Dispatch
Invasive Group B Streptococcus Infections Caused by Hypervirulent Clone of S. agalactiae Sequence Type 283, Hong Kong, China, 20211
Abstract
During September–October 2021, group B Streptococcus bloodstream infections surged among patients hospitalized in Hong Kong. Of 95 cases, 57 were caused by the hypervirulent strain sequence type 283, which at the time was also found in freshwater fish and wet market environments and thus poses a transmission threat.
In 2015, the zoonotic potential of group B Streptococcus (GBS) sequence type (ST) 283 was highlighted in the Singapore outbreak of bacteremia cases associated with consumption of raw freshwater fish, which led to the ban of raw freshwater fish in all ready-to-eat raw fish dishes in Singapore (1,2). ST283 was found not only among patients with GBS bacteremia in Southeast Asia but also associated with aquaculture (2–4). ST283 was first noted to cause infection in humans in the mid-1990s and its invasiveness was described in meningitis and bacteremia cases in 2000 and 2006 respectively (5,6), and its invasiveness was described. Since then, outbreaks among humans in Singapore and among freshwater fish species in Southeast Asia and Brazil have been noted (3,4,7,8). During September–October 2021, a surge of ST283 invasive GBS (iGBS) disease among nonpregnant adults was reported in public hospitals in Hong Kong, China, in response to which the Centre for Health Protection (CHP) issued a special bulletin on the investigation and heightened surveillance of the group B Streptococcus invasive disease (9). Because consumption of raw freshwater fish is prohibited in Hong Kong, other potential sources or transmission routes of the strain were investigated. We report the molecular epidemiology of GBS ST283 and the clinical characteristics of infections during that period.
During September 2–November 6 (weeks 35–44) of 2021, a total of 95 cases of iGBS infections were reported from 17 public hospitals across Hong Kong. GBS isolates were characterized by whole-genome sequencing. In addition, 11 GBS strains were isolated from fish and environmental samples collected from wet markets at week 39. Clinical and laboratory data retrieval were approved by the Central Institutional Review Board of the Hospital Authority (reference no. CIRB2022-056-5) and the Joint New Territories East Cluster–Chinese University of Hong Kong Ethics Committee (reference no. CREC2018.509).
We confirmed the identity of GBS isolates by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonics, https://www.bruker.com) and extracted DNA by using the QIAGEN EZ1 Virus Mini Kit v2.0 (QIAGEN, https://www.qiagen.com) on the EZ1 Advanced XL platform according to the manufacturer’s protocol. We prepared libraries by using the Illumina Nextera XT DNA Library Preparation Kit (Illumina, https://www.illumina.com) according to instructions and performed sequencing by using an Illumina sequencer with an average of 60× coverage. The pipeline of genome assembly and matching of STs, antimicrobial resistance genes, and virulence factors have been previously described (10). We mapped assembled genomes to reference genome SG-M158 (GenBank accession no. CP021864) by using Snippy v4.6.0 (http://github.com). For comparison, we included archived ST283 genomes CU_GBS_98, CU_GBS_08 from Hong Kong and SG-M1 from Singapore (GenBank accession nos. CP010875.1, CP010874.1, and CP012419.2). Variants were called by Freebayes v1.3.6 (https://github.com/freebayes/freebayes), and sites of single-nucleotide polymorphisms (SNPs) were further analyzed. We identified recombination sites and filtered them by using Gubbins v3.1.0 (https://github.com/nickjcroucher.gubbins) and generated a whole-genome SNP tree by using IQ-TREE v2.2.0.3 (https://github.com/iqtree/iqtree2) and autoselected model (TVMe+ASC+R2). Branch support was provided by UFBoot with >1,000 iterations. Sequence data of the strains are available under National Center for Biotechnology Information BioProject no. PRJNA999453.
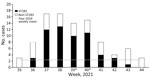
The number of GBS bacteremia cases surged during weeks 37–40, when 14–17 cases per week were reported (Figure 1), which was 4-fold higher than baseline in 2019. During weeks 36–43, a total of 57 (60%) cases belonged to ST283, and the last case of ST283 infection was observed in week 43. The mean age for the overall iGBS cohort was 66.7 ± 17.8 years (range 1 month–96 years), and ST283 cases were limited to nonpregnant adults (age range 31–90 years, mean 66.2 ± 12 years) (Table). The male:female ratio was the same for patients in non-ST283 and ST283 cohorts, and mortality rates were 7.9% (3/38) for patients with non-ST283 infection and 8.8% (5/57) for patients with ST283 infection. Joint infections with involvement of single or multiple joints was common in patients with ST283 infection (26.3%) (p = 0.02). We found no statistically significant difference in the number of comorbidities between cohorts with ST283 and those with non-ST283 infections. During the study period, other STs found in patients with iGBS infection were ST1, ST17, ST890, and ST12. Among the 11 nonhuman GBS isolates, 3 fish strains belonged to serotype Ia ST7, and 8 were ST283. Both ST7 and 283 have been associated with disease in fish (4,5,8,10), suggesting that they were present in food animals before harvest rather than contaminated after harvest.
Antibiotic susceptibility testing following Clinical Laboratory and Standards Institute guidelines indicated that all GBS strains were sensitive to penicillin (11). Genome analysis showed that the ST283 isolates had 0–1037 SNPs with an average distance of 484 SNPs. Two clades of ST283 were depicted by the presence of the TetM gene (Figure 2, panel A). The main clade (cluster I), which consisted of 57 isolates (including 3 from fish, 4 from wet market environment, and 1 from tank water), had no antimicrobial resistant genes and clustered with SG-M1. Among the 57 isolates, 33 (67%) of 49 were from patients who had a history of handling raw fish, and that cluster led to the upsurge of cases in hospitals. A minor clade of 7 ST283 strains (cluster II) carried the tetM gene on Tn916 and clustered with archived genomes (CU_GBS_98 and CU_GBS_08) along with a ST739 strain (a single-locus variant of ST283 at the adhP gene). Compared with the archived genomes and ST739, those strains also lacked lmb and scpB genes. Lmb encodes for laminin-binding protein and scpB for part of the pilus island for invasion to host epithelial cells. We compared the 2 clusters with 303 ST283 genomes from the National Center for Biotechnology Information (Appendix 1). Cluster I was a separate clade from the Singapore outbreak and showed convergence to strains from Thailand (Appendix 2). Cluster II was also observed in species of fish in Southeast Asia (4,5,8).
According to the Hong Kong Observatory, the mean ambient temperatures were 29.7°C in September and 26°C in October 2021. September was one of the hottest months of the year, which concurred with previous findings of higher prevalence rates of GBS isolation from food animals and iGBS disease caused by ST283 in patients during the summer, resulting from the high mean temperature (>28°C) (10,12). CHP issued a special bulletin with regard to the ST283 upsurge, when a history of handling raw fish was noted. Consumption of raw fish from dining outlets could be ruled out because selling raw freshwater fish sashimi had been prohibited in Hong Kong for >30 years (9). Two of the case-patients were chefs, 1 of whom recalled having a minor puncture wound while cleaning grass carp ≈1 week before hospital admission. Another case involved a part-time fishmonger. Zoonotic Streptococcus iniae infection after handling raw fish, especially by persons with a puncture wound, was previously noted in Hong Kong (13,14). Thus, contact with raw fish may also be a transmission route for iGBS infection. The CHP introduced public health measures to enhance proper handling of raw fish and advised persons to thoroughly cook freshwater fish (9).
We report a cluster of invasive GBS ST283 infections in nonpregnant adults in the late summer of 2021 and found the same ST in freshwater fish and environmental samples in wet markets of Hong Kong during that period. Because selling raw freshwater fish sashimi is prohibited locally, the main association of the upsurge was contact with or improper handling of freshwater fish, highlighting the zoonotic potential of GBS ST283 transmission through contact with freshwater fish.
Dr. Li is a scientific officer in the Department of Microbiology, Chinese University of Hong Kong. Her interests include bacterial infectious diseases and antimicrobial resistance in human and animals from a One Health perspective and assay development for rapid detection of bacterial identification and typing.
Dr. Tse is a clinical microbiologist at Khoo Teck Puat Hospital, Singapore. His research interests include health informatics and microbial genomics.
Acknowledgments
We sincerely thank and acknowledge members of the Communicable Diseases Branch of the CHP, Hong Kong Special Administrative Region, China, who were involved in the coordination and epidemiologic investigations of the upsurge. We also thank the Microbiology Division of the Public Health Laboratory Services for their contribution to our investigation.
The study is partially supported by a Bacterial Surveillance Fund (to M.I.) in the Department of Microbiology at the Chinese University of Hong Kong.
C.L., H.T., and C.Z. were involved in laboratory work, data and genome analyses, and first draft of the manuscript. G.K.Y.C., A.L.H.L., D.T.Y.H., C.C., S.K.Y.C., J.L., K.L., T.L.Q., K.S.C.F., C.T., S.C.Y.W., V.C.Y.C., D.C.L., and M.I. were involved in microbiological management of the bacteremia cases, data collection, and provision of bacterial strains. C.Z., C.L., N.W.S.L., and J.Y. provided technical support for laboratory processing, typing of GBS strains, and sequencing preparation. D.C.L. coordinated the project with C.L., supervised by M.I. D.C.L., H.T., C.L., and M.I. prepared the final version of the manuscript. All authors agreed, read, and contributed to the submitted version of the manuscript. All authors have no conflicts of interest to declare.
References
- Tan S, Lin Y, Foo K, Koh HF, Tow C, Zhang Y, et al. Group B Streptococcus serotype III sequence type 283 bacteremia associated with consumption of raw fish, Singapore. Emerg Infect Dis. 2016;22:1970–3. DOIGoogle Scholar
- Chau ML, Chen SL, Yap M, Hartantyo SHP, Chiew PKT, Fernandez CJ, et al. Group B Streptococcus infections caused by improper sourcing and handling of fish for raw consumption, Singapore, 2015–2016. Emerg Infect Dis. 2017;23:2002–10. DOIPubMedGoogle Scholar
- Barkham T, Zadoks RN, Azmai MNA, Baker S, Bich VTN, Chalker V, et al. One hypervirulent clone, sequence type 283, accounts for a large proportion of invasive Streptococcus agalactiae isolated from humans and diseased tilapia in Southeast Asia. PLoS Negl Trop Dis. 2019;13:e0007421. DOIGoogle Scholar
- Sirimanapong W, Phước NN, Crestani C, Chen S, Zadoks RN. Geographical, temporal and host-species distribution of potentially human-pathogenic group B Streptococcus in aquaculture species in Southeast Asia. Pathogens. 2023;12:525. DOIPubMedGoogle Scholar
- Wilder-Smith E, Chow KM, Kay R, Ip M, Tee N. Group B streptococcal meningitis in adults: recent increase in Southeast Asia. Aust N Z J Med. 2000;30:462–5. DOIPubMedGoogle Scholar
- Ip M, Cheuk ES, Tsui MH, Kong F, Leung TN, Gilbert GL. Identification of a Streptococcus agalactiae serotype III subtype 4 clone in association with adult invasive disease in Hong Kong. J Clin Microbiol. 2006;44:4252–4. DOIPubMedGoogle Scholar
- Singapore Ministry of Health. Advisory on the increase in the number of invasive group B Streptococcus cases [cited 2023 Nov 15]. https://www.moh.gov.sg/news-highlights/details/advisory-on-the-increase-in-the-number-of-invasive-group-b-streptococcus-cases.
- Leal CAG, Queiroz GA, Pereira FL, Tavares GC, Figueiredo HCP. Streptococcus agalactiae sequence type 283 in farmed fish, Brazil. Emerg Infect Dis. 2019;25:776–9. DOIPubMedGoogle Scholar
- Centre for Health Protection. Cluster of invasive group B Streptococcus ST283 cases related to freshwater fish 2021 [cited 2023 Nov 15]. https://www.chp.gov.hk/files/pdf/cdw_v18_sp_bulletin.pdf
- Sapugahawatte DN, Li C, Dharmaratne P, Zhu C, Yeoh YK, Yang J, et al. Prevalence and characteristics of Streptococcus agalactiae from freshwater fish and pork in Hong Kong wet markets. Antibiotics (Basel). 2022;11:397. DOIPubMedGoogle Scholar
- Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 31st edition (M100). Wayne (PA): The Institute; 2021.
- Ip M, Ang I, Fung K, Liyanapathirana V, Luo MJ, Lai R. Hypervirulent clone of group B Streptococcus serotype III sequence type 283, Hong Kong, 1993–2012. Emerg Infect Dis. 2016;22:1800–3. DOIPubMedGoogle Scholar
- Lau SK, Woo PC, Luk WK, Fung AM, Hui WT, Fong AH, et al. Clinical isolates of Streptococcus iniae from Asia are more mucoid and beta-hemolytic than those from North America. Diagn Microbiol Infect Dis. 2006;54:177–81. DOIPubMedGoogle Scholar
- Agnew W, Barnes AC. Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet Microbiol. 2007;122:1–15. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: December 11, 2024
1Preliminary results from this study were presented at the 3rd ISSAD (International Symposium on ‘Streptococcus agalactiae’ Disease); October 16–18, 2023; Rio de Janeiro, Brazil.
2These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 1—January 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Margaret Ip, Chinese University of Hong Kong, Rm 38051, 1/F, Department of Microbiology, Lui Chee Wo Clinical Sciences Bldg, Prince of Wales Hospital, Shatin New Territories, Hong Kong, China
Top