
Amniocentesis is a medical procedure used primarily in the prenatal diagnosis of genetic conditions. It has other uses such as in the assessment of infection and fetal lung maturity. Prenatal diagnostic testing, which includes amniocentesis, is necessary to conclusively diagnose the majority of genetic disorders, with amniocentesis being the gold-standard procedure after 15 weeks' gestation.

The amnion is a membrane that closely covers human and various other embryos when they first form. It fills with amniotic fluid, which causes the amnion to expand and become the amniotic sac that provides a protective environment for the developing embryo. The amnion, along with the chorion, the yolk sac and the allantois protect the embryo. In birds, reptiles and monotremes, the protective sac is enclosed in a shell. In marsupials and placental mammals, it is enclosed in a uterus.

The chorion is the outermost fetal membrane around the embryo in mammals, birds and reptiles (amniotes). It develops from an outer fold on the surface of the yolk sac, which lies outside the zona pellucida, known as the vitelline membrane in other animals. In insects, it is developed by the follicle cells while the egg is in the ovary. Some mollusks also have chorions as part of their eggs. For example, fragile octopus eggs have only a chorion as their envelope.
Oligohydramnios is a medical condition in pregnancy characterized by a deficiency of amniotic fluid, the fluid that surrounds the fetus in the abdomen, in the amniotic sac. The limiting case is anhydramnios, where there is a complete absence of amniotic fluid. It is typically diagnosed by ultrasound when the amniotic fluid index (AFI) measures less than 5 cm or when the single deepest pocket (SDP) of amniotic fluid measures less than 2 cm. Amniotic fluid is necessary to allow for normal fetal movement, lung development, and cushioning from uterine compression. Low amniotic fluid can be attributed to a maternal, fetal, placental or idiopathic cause and can result in poor fetal outcomes including death. The prognosis of the fetus is dependent on the etiology, gestational age at diagnosis, and the severity of the oligohydramnios.
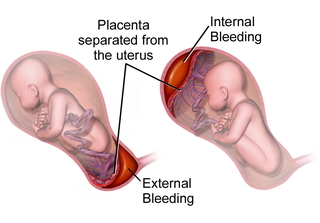
Placental abruption is when the placenta separates early from the uterus, in other words separates before childbirth. It occurs most commonly around 25 weeks of pregnancy. Symptoms may include vaginal bleeding, lower abdominal pain, and dangerously low blood pressure. Complications for the mother can include disseminated intravascular coagulopathy and kidney failure. Complications for the baby can include fetal distress, low birthweight, preterm delivery, and stillbirth.
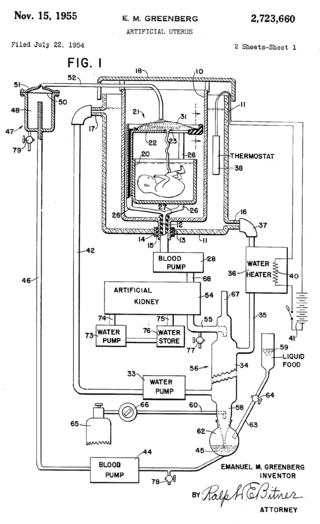
An artificial womb or artificial uterus is a device that would allow for extracorporeal pregnancy, by growing a fetus outside the body of an organism that would normally carry the fetus to term. An artificial uterus, as a replacement organ, would have many applications. It could be used to assist male or female couples in the development of a fetus. This can potentially be performed as a switch from a natural uterus to an artificial uterus, thereby moving the threshold of fetal viability to a much earlier stage of pregnancy. In this sense, it can be regarded as a neonatal incubator with very extended functions. It could also be used for the initiation of fetal development. An artificial uterus could also help make fetal surgery procedures at an early stage an option instead of having to postpone them until term of pregnancy.
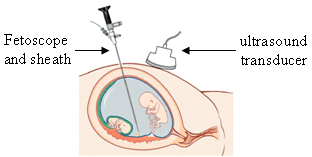
Fetoscopy is an endoscopic procedure during pregnancy to allow surgical access to the fetus, the amniotic cavity, the umbilical cord, and the fetal side of the placenta. A small (3–4 mm) incision is made in the abdomen, and an endoscope is inserted through the abdominal wall and uterus into the amniotic cavity. Fetoscopy allows for medical interventions such as a biopsy or a laser occlusion of abnormal blood vessels or the treatment of spina bifida.

Hydrops fetalis or hydrops foetalis is a condition in the fetus characterized by an accumulation of fluid, or edema, in at least two fetal compartments. By comparison, hydrops allantois or hydrops amnion is an accumulation of excessive fluid in the allantoic or amniotic space, respectively.

The EXIT procedure, or ex utero intrapartum treatment procedure, is a specialized surgical delivery procedure used to deliver babies who have airway compression. Causes of airway compression in newborn babies result from a number of rare congenital disorders, including bronchopulmonary sequestration, congenital cystic adenomatoid malformation, mouth or neck tumor such as teratoma, and lung or pleural tumor such as pleuropulmonary blastoma. Airway compression discovered at birth is a medical emergency. In many cases, however, the airway compression is discovered during prenatal ultrasound exams, permitting time to plan a safe delivery using the EXIT procedure or other means.
Placental insufficiency or utero-placental insufficiency is the failure of the placenta to deliver sufficient nutrients to the fetus during pregnancy, and is often a result of insufficient blood flow to the placenta. The term is also sometimes used to designate late decelerations of fetal heart rate as measured by cardiotocography or an NST, even if there is no other evidence of reduced blood flow to the placenta, normal uterine blood flow rate being 600mL/min.

Percutaneous umbilical cord blood sampling (PUBS), also called cordocentesis, fetal blood sampling, or umbilical vein sampling is a diagnostic genetic test that examines blood from the fetal umbilical cord to detect fetal abnormalities. Fetal and maternal blood supply are typically connected in utero with one vein and two arteries to the fetus. The umbilical vein is responsible for delivering oxygen rich blood to the fetus from the mother; the umbilical arteries are responsible for removing oxygen poor blood from the fetus. This allows for the fetus’ tissues to properly perfuse. PUBS provides a means of rapid chromosome analysis and is useful when information cannot be obtained through amniocentesis, chorionic villus sampling, or ultrasound ; this test carries a significant risk of complication and is typically reserved for pregnancies determined to be at high risk for genetic defect. It has been used with mothers with immune thrombocytopenic purpura.

Velamentous cord insertion is a complication of pregnancy where the umbilical cord is inserted in the fetal membranes. It is a major cause of antepartum hemorrhage that leads to loss of fetal blood and associated with high perinatal mortality. In normal pregnancies, the umbilical cord inserts into the middle of the placental mass and is completely encased by the amniotic sac. The vessels are hence normally protected by Wharton's jelly, which prevents rupture during pregnancy and labor. In velamentous cord insertion, the vessels of the umbilical cord are improperly inserted in the chorioamniotic membrane, and hence the vessels traverse between the amnion and the chorion towards the placenta. Without Wharton's jelly protecting the vessels, the exposed vessels are susceptible to compression and rupture.

Twin reversed arterial perfusion sequence, also called TRAP sequence, TRAPS, or acardiac twinning, is a rare complication of monochorionic twin pregnancies. It is a severe variant of twin-to-twin transfusion syndrome (TTTS). In addition to the twins' blood systems being connected instead of independent, one twin, called the acardiac twin, TRAP fetus or acardius, is severely malformed. The heart is missing or deformed, hence the name "acardiac", as are the upper structures of the body. The legs may be partially present or missing, and internal structures of the torso are often poorly formed. The other twin is usually normal in appearance. The normal twin, called the pump twin, drives blood through both fetuses. It is called "reversed arterial perfusion" because in the acardiac twin the blood flows in a reversed direction.
An obstetric labor complication is a difficulty or abnormality that arises during the process of labor or delivery.
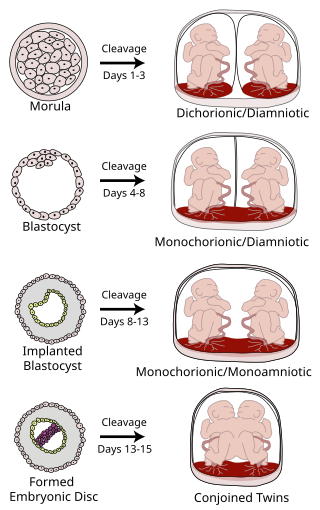
Monoamniotic twins are identical or semi-identical twins that share the same amniotic sac within their mother's uterus. Monoamniotic twins are always monochorionic and are usually termed Monoamniotic-Monochorionic twins. They share the placenta, but have two separate umbilical cords. Monoamniotic twins develop when an embryo does not split until after formation of the amniotic sac, at about 9–13 days after fertilization. Monoamniotic triplets or other monoamniotic multiples are possible, but extremely rare. Other obscure possibilities include multiples sets where monoamniotic twins are part of a larger gestation such as triplets, quadruplets, or more.

Monochorionic twins are monozygotic (identical) twins that share the same placenta. If the placenta is shared by more than two twins, these are monochorionic multiples. Monochorionic twins occur in 0.3% of all pregnancies. Seventy-five percent of monozygotic twin pregnancies are monochorionic; the remaining 25% are dichorionic diamniotic. If the placenta divides, this takes place before the third day after fertilization.
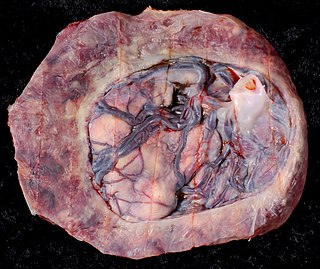
Circumvallate placenta is a rare condition affecting about 1-2% of pregnancies, in which the amnion and chorion fetal membranes essentially "double back" on the fetal side around the edges of the placenta. After delivery, a circumvallate placenta has a thick ring of membranes on its fetal surface. Circumvallate placenta is a placental morphological abnormality associated with increased fetal morbidity and mortality due to the restricted availability of nutrients and oxygen to the developing fetus.
Amnioinfusion is a method in which isotonic fluid is instilled into the uterine cavity.
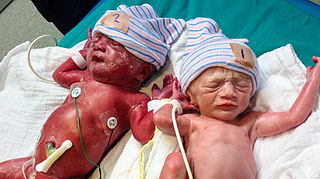
Twin anemia-polycythemia sequence (TAPS) is a chronic type of unbalanced fetal transfusion in monochorionic twins that results in polycythemia in the TAPS recipient and anemia in the TAPS donor due to tiny placental anastomoses. Post-laser TAPS and spontaneous TAPS are the two forms of TAPS. Unlike twin-to-twin transfusion syndrome, which arises when twin oligohydramnios polyhydramnios sequence (TOPS) is present, TAPS develops in its absence.
An Intrauterine transfusion (IUT) is a procedure that provides blood to a fetus, most commonly through the umbilical cord. It is used in cases of severe fetal anemia, such as when fetal red blood cells are being destroyed by maternal antibodies, or parvovirus B19 infection, homozygous alpha-thalassemia, or twin-to-twin transfusion syndrome. IUTs are performed by perinatologists at hospitals or specialized centers.
















