
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets (thrombocytes) and fibrin to form a blood clot to prevent blood loss. Even when a blood vessel is not injured, blood clots may form in the body under certain conditions. A clot, or a piece of the clot, that breaks free and begins to travel around the body is known as an embolus.
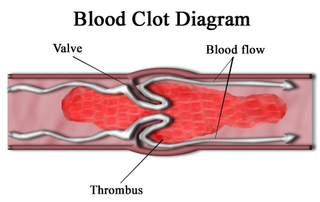
A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to stop and prevent further bleeding, but can be harmful in thrombosis, when a clot obstructs blood flow through healthy blood vessels in the circulatory system.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.

Ischemia or ischaemia is a restriction in blood supply to any tissues, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism. Ischemia is generally caused by problems with blood vessels, with resultant damage to or dysfunction of tissue i.e. hypoxia and microvascular dysfunction. It also means local hypoxia in a given part of a body sometimes resulting from constriction. Ischemia comprises not only insufficiency of oxygen, but also reduced availability of nutrients and inadequate removal of metabolic wastes. Ischemia can be partial or total blockage. The inadequate delivery of oxygenated blood to the organs must be resolved either by treating the cause of the inadequate delivery or reducing the oxygen demand of the system that needs it. For example, patients with myocardial ischemia have a decreased blood flow to the heart and are prescribed with medications that reduce chronotrophy and ionotrophy to meet the new level of blood delivery supplied by the stenosed so that it is adequate.
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.

Streptokinase (SK) is a thrombolytic medication and enzyme. As a medication it is used to break down clots in some cases of myocardial infarction, pulmonary embolism, and arterial thromboembolism. The type of heart attack it is used in is an ST elevation myocardial infarction (STEMI). It is given by injection into a vein.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.

Alteplase (t-PA), a biosynthetic form of human tissue-type plasminogen activator (t-PA), is a thrombolytic medication, used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. It is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.
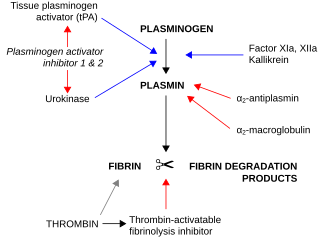
Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.

A cerebral infarction is the pathologic process that results in an area of necrotic tissue in the brain. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia), most commonly due to thromboembolism, and manifests clinically as ischemic stroke. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.
Anistreplase is a thrombolytic drug. It is also known as anisoylated plasminogen streptokinase activator complex (APSAC). As a thrombolytic drug, it is used to treat blood clots in emergency situations.
Tenecteplase, sold under the trade names TNKase, Metalyse and Elaxim, is an enzyme used as a thrombolytic drug.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.
The fibrinolysis system is responsible for removing blood clots. Hyperfibrinolysis describes a situation with markedly enhanced fibrinolytic activity, resulting in increased, sometimes catastrophic bleeding. Hyperfibrinolysis can be caused by acquired or congenital reasons. Among the congenital conditions for hyperfibrinolysis, deficiency of alpha-2-antiplasmin or plasminogen activator inhibitor type 1 (PAI-1) are very rare. The affected individuals show a hemophilia-like bleeding phenotype. Acquired hyperfibrinolysis is found in liver disease, in patients with severe trauma, during major surgical procedures, and other conditions. A special situation with temporarily enhanced fibrinolysis is thrombolytic therapy with drugs which activate plasminogen, e.g. for use in acute ischemic events or in patients with stroke. In patients with severe trauma, hyperfibrinolysis is associated with poor outcome. Moreover, hyperfibrinolysis may be associated with blood brain barrier impairment, a plasmin-dependent effect due to an increased generation of bradykinin.

Acute limb ischaemia (ALI) occurs when there is a sudden lack of blood flow to a limb.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon to open up the artery, with the possible additional use of one or more stents. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.
This article describes disparities existing between men and women in accessing and receiving care for a stroke. This article also describes factors outside of the health care system which contribute to this disparity.
Thrombus perviousness is an imaging biomarker which is used to estimate clot permeability from CT imaging. It reflects the ability of artery-occluding thrombi to let fluid seep into and through them. The more pervious a thrombus, the more fluid it lets through. Thrombus perviousness can be measured using radiological imaging routinely performed in the clinical management of acute ischemic stroke: CT scans without intravenous contrast combined with CT scans after intravenously administered contrast fluid. Pervious thrombi may let more blood pass through to the ischemic brain tissue, and/or have a larger contact surface and histopathology more sensitive for thrombolytic medication. Thus, patients with pervious thrombi may have less brain tissue damage by stroke. The value of thrombus perviousness in acute ischemic stroke treatment is currently being researched.






























