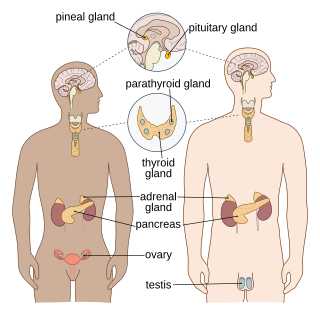
The endocrine system is a messenger system in an organism comprising feedback loops of hormones that are released by internal glands directly into the circulatory system and that target and regulate distant organs. In vertebrates, the hypothalamus is the neural control center for all endocrine systems.

Somatostatin, also known as growth hormone-inhibiting hormone (GHIH) or by several other names, is a peptide hormone that regulates the endocrine system and affects neurotransmission and cell proliferation via interaction with G protein-coupled somatostatin receptors and inhibition of the release of numerous secondary hormones. Somatostatin inhibits insulin and glucagon secretion.

Pituitary adenomas are tumors that occur in the pituitary gland. Most pituitary tumors are benign, approximately 35% are invasive and just 0.1% to 0.2% are carcinomas. Pituitary adenomas represent from 10% to 25% of all intracranial neoplasms and the estimated prevalence rate in the general population is approximately 17%.
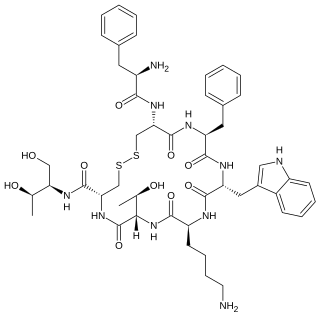
Octreotide, sold under the brand name Sandostatin among others, is an octapeptide that mimics natural somatostatin pharmacologically, though it is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone. It was first synthesized in 1979 by the chemist Wilfried Bauer, and binds predominantly to the somatostatin receptors SSTR2 and SSTR5.

Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen (17β-estradiol). Two classes of ER exist: nuclear estrogen receptors, which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors. This article refers to the former (ER).
Growth hormone–releasing hormone (GHRH), also known as somatocrinin among other names in its endogenous form and as somatorelin (INN) in its pharmaceutical form, is a releasing hormone of growth hormone (GH). It is a 44-amino acid peptide hormone produced in the arcuate nucleus of the hypothalamus.
The prolactin receptor (PRLR) is a type I cytokine receptor encoded in humans by the PRLR gene on chromosome 5p13-14. It is the receptor for prolactin (PRL). The PRLR can also bind to and be activated by growth hormone (GH) and human placental lactogen (hPL). The PRLR is expressed in the mammary glands, pituitary gland, and other tissues. It plays an important role in lobuloalveolar development of the mammary glands during pregnancy and in lactation.

Neuroendocrine tumors (NETs) are neoplasms that arise from cells of the endocrine (hormonal) and nervous systems. They most commonly occur in the intestine, where they are often called carcinoid tumors, but they are also found in the pancreas, lung, and the rest of the body.
Somatostatinomas are a tumor of the delta cells of the endocrine pancreas that produces somatostatin. Increased levels of somatostatin inhibit pancreatic hormones and gastrointestinal hormones. Thus, somatostatinomas are associated with mild diabetes mellitus, steatorrhoea and gallstones, and achlorhydria. Somatostatinomas are commonly found in the head of pancreas. Only ten percent of somatostatinomas are functional tumours [9], and 60–70% of tumours are malignant. Nearly two-thirds of patients with malignant somatostatinomas will present with metastatic disease.

Enteroendocrine cells are specialized cells of the gastrointestinal tract and pancreas with endocrine function. They produce gastrointestinal hormones or peptides in response to various stimuli and release them into the bloodstream for systemic effect, diffuse them as local messengers, or transmit them to the enteric nervous system to activate nervous responses. Enteroendocrine cells of the intestine are the most numerous endocrine cells of the body. They constitute an enteric endocrine system as a subset of the endocrine system just as the enteric nervous system is a subset of the nervous system. In a sense they are known to act as chemoreceptors, initiating digestive actions and detecting harmful substances and initiating protective responses. Enteroendocrine cells are located in the stomach, in the intestine and in the pancreas. Microbiota play key roles in the intestinal immune and metabolic responses in these enteroendocrine cells via their fermentation product, acetate.
G protein-coupled estrogen receptor 1 (GPER), also known as G protein-coupled receptor 30 (GPR30), is a protein that in humans is encoded by the GPER gene. GPER binds to and is activated by the female sex hormone estradiol and is responsible for some of the rapid effects that estradiol has on cells.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.
Somatostatin receptor type 5 is a protein that in humans is encoded by the SSTR5 gene.

Somatostatin receptor type 1 is a protein that in humans is encoded by the SSTR1 gene.
Shekel Somatostatin receptor type 3 is a protein that in humans is encoded by the SSTR3 gene.

Somatostatin receptor type 4 is a protein that in humans is encoded by the SSTR4 gene.

Securin is a protein that in humans is encoded by the PTTG1 gene.
Membrane progesterone receptors (mPRs) are a group of cell surface receptors and membrane steroid receptors belonging to the progestin and adipoQ receptor (PAQR) family which bind the endogenous progestogen and neurosteroid progesterone, as well as the neurosteroid allopregnanolone. Unlike the progesterone receptor (PR), a nuclear receptor which mediates its effects via genomic mechanisms, mPRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The mPRs mediate important physiological functions in male and female reproductive tracts, liver, neuroendocrine tissues, and the immune system as well as in breast and ovarian cancer.

Somatostatin receptor antagonists are a class of chemical compounds that work by imitating the structure of the neuropeptide somatostatin. The somatostatin receptors are G protein-coupled receptors. Somatostatin receptor subtypes in humans are sstr1, 2A, 2 B, 3, 4 and 5. While normally expressed in the gastrointestinal (GI) tract, pancreas, hypothalamus, and central nervous system (CNS), they are expressed in different types of tumours. The predominant subtype in cancer cells is the ssrt2 subtype, which is expressed in neuroblastomas, meningiomas, medulloblastomas, breast carcinomas, lymphomas, renal cell carcinomas, paragangliomas, small cell lung carcinomas and hepatocellular carcinomas.

Somatostatin receptor antagonists are a class of chemical compounds that work by imitating the structure of the neuropeptide somatostatin, which is an endogenous hormone found in the human body. The somatostatin receptors are G protein-coupled receptors. Somatostatin receptor subtypes in humans include sstr1, 2A, 2 B, 3, 4, and 5. While normally expressed in the gastrointestinal (GI) tract, pancreas, hypothalamus, and central nervous system (CNS), they are expressed in different types of tumours. The predominant subtype in cancer cells is the ssrt2 subtype, which is expressed in neuroblastomas, meningiomas, medulloblastomas, breast carcinomas, lymphomas, renal cell carcinomas, paragangliomas, small cell lung carcinomas, and hepatocellular carcinomas.





















