
Stromatolites or stromatoliths are layered sedimentary formations (microbialite) that are created mainly by photosynthetic microorganisms such as cyanobacteria, sulfate-reducing bacteria, and Pseudomonadota. These microorganisms produce adhesive compounds that cement sand and other rocky materials to form mineral "microbial mats". In turn, these mats build up layer by layer, growing gradually over time.

Cyanobacteria, also called Cyanobacteriota or Cyanophyta, are a phylum of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" refers to their bluish green (cyan) color, which forms the basis of cyanobacteria's informal common name, blue-green algae, although as prokaryotes they are not scientifically classified as algae.

Geomicrobiology is the scientific field at the intersection of geology and microbiology and is a major subfield of geobiology. It concerns the role of microbes on geological and geochemical processes and effects of minerals and metals to microbial growth, activity and survival. Such interactions occur in the geosphere, the atmosphere and the hydrosphere. Geomicrobiology studies microorganisms that are driving the Earth's biogeochemical cycles, mediating mineral precipitation and dissolution, and sorbing and concentrating metals. The applications include for example bioremediation, mining, climate change mitigation and public drinking water supplies.
The purple sulfur bacteria (PSB) are part of a group of Pseudomonadota capable of photosynthesis, collectively referred to as purple bacteria. They are anaerobic or microaerophilic, and are often found in stratified water environments including hot springs, stagnant water bodies, as well as microbial mats in intertidal zones. Unlike plants, algae, and cyanobacteria, purple sulfur bacteria do not use water as their reducing agent, and therefore do not produce oxygen. Instead, they can use sulfur in the form of sulfide, or thiosulfate (as well, some species can use H2, Fe2+, or NO2−) as the electron donor in their photosynthetic pathways. The sulfur is oxidized to produce granules of elemental sulfur. This, in turn, may be oxidized to form sulfuric acid.
Heliobacteria are a unique subset of prokaryotic bacteria that process light for energy. Distinguishable from other phototrophic bacteria, they utilize a unique photosynthetic pigment, bacteriochlorophyll g and are the only known Gram-positive phototroph. They are a key player in symbiotic nitrogen fixation alongside plants, and use a type I reaction center like green-sulfur bacteria.
Photoheterotrophs are heterotrophic phototrophs—that is, they are organisms that use light for energy, but cannot use carbon dioxide as their sole carbon source. Consequently, they use organic compounds from the environment to satisfy their carbon requirements; these compounds include carbohydrates, fatty acids, and alcohols. Examples of photoheterotrophic organisms include purple non-sulfur bacteria, green non-sulfur bacteria, and heliobacteria. These microorganisms are ubiquitous in aquatic habitats, occupy unique niche-spaces, and contribute to global biogeochemical cycling. Recent research has also indicated that the oriental hornet and some aphids may be able to use light to supplement their energy supply.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
The microbial food web refers to the combined trophic interactions among microbes in aquatic environments. These microbes include viruses, bacteria, algae, heterotrophic protists. In aquatic ecosystems, microbial food webs are essential because they form the basis for the cycling of nutrients and energy. These webs are vital to the stability and production of ecosystems in a variety of aquatic environments, including lakes, rivers, and oceans. By converting dissolved organic carbon (DOC) and other nutrients into biomass that larger organisms may eat, microbial food webs maintain higher trophic levels. Thus, these webs are crucial for energy flow and nutrient cycling in both freshwater and marine ecosystems.

A microbial mat is a multi-layered sheet or biofilm of microbial colonies, composed of mainly bacteria and/or archaea. Microbial mats grow at interfaces between different types of material, mostly on submerged or moist surfaces, but a few survive in deserts. A few are found as endosymbionts of animals.

Lonar Lake, also known as Lonar crater, is a saline, soda lake, located at Lonar, 79 km from Buldhana city in Buldhana district, Maharashtra, India. It is a notified National Geo-heritage Monument.
Sulfur is metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur can have an oxidation state from -2 to +6 and is reduced or oxidized by a diverse range of organisms. The element is present in proteins, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes.
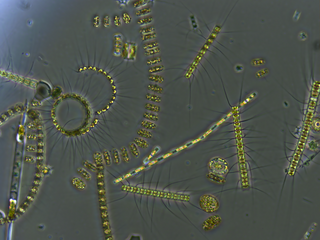
Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλανκτος, meaning "wanderer" or "drifter", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and freshwater.

Marine microorganisms are defined by their habitat as microorganisms living in a marine environment, that is, in the saltwater of a sea or ocean or the brackish water of a coastal estuary. A microorganism is any microscopic living organism or virus, which is invisibly small to the unaided human eye without magnification. Microorganisms are very diverse. They can be single-celled or multicellular and include bacteria, archaea, viruses, and most protozoa, as well as some fungi, algae, and animals, such as rotifers and copepods. Many macroscopic animals and plants have microscopic juvenile stages. Some microbiologists also classify viruses as microorganisms, but others consider these as non-living.
Soil microbiology is the study of microorganisms in soil, their functions, and how they affect soil properties. It is believed that between two and four billion years ago, the first ancient bacteria and microorganisms came about on Earth's oceans. These bacteria could fix nitrogen, in time multiplied, and as a result released oxygen into the atmosphere. This led to more advanced microorganisms, which are important because they affect soil structure and fertility. Soil microorganisms can be classified as bacteria, actinomycetes, fungi, algae and protozoa. Each of these groups has characteristics that define them and their functions in soil.

Microbiologically induced calcium carbonate precipitation (MICP) is a bio-geochemical process that induces calcium carbonate precipitation within the soil matrix. Biomineralization in the form of calcium carbonate precipitation can be traced back to the Precambrian period. Calcium carbonate can be precipitated in three polymorphic forms, which in the order of their usual stabilities are calcite, aragonite and vaterite. The main groups of microorganisms that can induce the carbonate precipitation are photosynthetic microorganisms such as cyanobacteria and microalgae; sulfate-reducing bacteria; and some species of microorganisms involved in nitrogen cycle. Several mechanisms have been identified by which bacteria can induce the calcium carbonate precipitation, including urea hydrolysis, denitrification, sulfate production, and iron reduction. Two different pathways, or autotrophic and heterotrophic pathways, through which calcium carbonate is produced have been identified. There are three autotrophic pathways, which all result in depletion of carbon dioxide and favouring calcium carbonate precipitation. In heterotrophic pathway, two metabolic cycles can be involved: the nitrogen cycle and the sulfur cycle. Several applications of this process have been proposed, such as remediation of cracks and corrosion prevention in concrete, biogrout, sequestration of radionuclides and heavy metals.
Arsenate-reducing bacteria are bacteria which reduce arsenates. Arsenate-reducing bacteria are ubiquitous in arsenic-contaminated groundwater (aqueous environment). Arsenates are salts or esters of arsenic acid (H3AsO4), consisting of the ion AsO43−. They are moderate oxidizers that can be reduced to arsenites and to arsine. Arsenate can serve as a respiratory electron acceptor for oxidation of organic substrates and H2S or H2. Arsenates occur naturally in minerals such as adamite, alarsite, legrandite, and erythrite, and as hydrated or anhydrous arsenates. Arsenates are similar to phosphates since arsenic (As) and phosphorus (P) occur in group 15 (or VA) of the periodic table. Unlike phosphates, arsenates are not readily lost from minerals due to weathering. They are the predominant form of inorganic arsenic in aqueous aerobic environments. On the other hand, arsenite is more common in anaerobic environments, more mobile, and more toxic than arsenate. Arsenite is 25–60 times more toxic and more mobile than arsenate under most environmental conditions. Arsenate can lead to poisoning, since it can replace inorganic phosphate in the glyceraldehyde-3-phosphate --> 1,3-biphosphoglycerate step of glycolysis, producing 1-arseno-3-phosphoglycerate instead. Although glycolysis continues, 1 ATP molecule is lost. Thus, arsenate is toxic due to its ability to uncouple glycolysis. Arsenate can also inhibit pyruvate conversion into acetyl-CoA, thereby blocking the TCA cycle, resulting in additional loss of ATP.

Microbialite is a benthic sedimentary deposit made of carbonate mud that is formed with the mediation of microbes. The constituent carbonate mud is a type of automicrite ; therefore, it precipitates in situ instead of being transported and deposited. Being formed in situ, a microbialite can be seen as a type of boundstone where reef builders are microbes, and precipitation of carbonate is biotically induced instead of forming tests, shells or skeletons.

Marine prokaryotes are marine bacteria and marine archaea. They are defined by their habitat as prokaryotes that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. All cellular life forms can be divided into prokaryotes and eukaryotes. Eukaryotes are organisms whose cells have a nucleus enclosed within membranes, whereas prokaryotes are the organisms that do not have a nucleus enclosed within a membrane. The three-domain system of classifying life adds another division: the prokaryotes are divided into two domains of life, the microscopic bacteria and the microscopic archaea, while everything else, the eukaryotes, become the third domain.

Laguna Negra is a lake in the Catamarca Province of Argentina. It lies on the Puna high plateau next to two other lakes and salt flats. The lake is less than 2 metres deep and forms a rough rectangle with a surface of 8.6 square kilometres (3.3 sq mi). Laguna Negra loses its water through evaporation, and is replenished through surface runoff and groundwater which ultimately originate to a large part from snowmelt. The waters of the lake are salty.
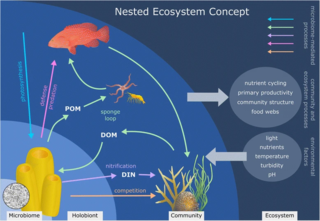
Sponge microbiomes are diverse communities of microorganisms in symbiotic association with marine sponges as their hosts. These microorganisms include bacteria, archaea, fungi, viruses, among others. The sponges have the ability to filter seawater and recycle nutrients while providing a safe habitat to many microorganisms, which provide the sponge host with fixed nitrogen and carbon, and stimulates the immune system. Together, a sponge and its microbiome form a holobiont, with a single sponge often containing more than 40 bacterial phyla, making sponge microbial environments a diverse and dense community. Furthermore, individual holobionts work hand in hand with other near holobionts becoming a nested ecosystem, affecting the environment at multiple scales.
























