Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients. ... [It] means integrating individual clinical expertise with the best available external clinical evidence from systematic research." The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.

Meta-analysis is a method of synthesis of quantitative data from multiple independent studies addressing a common research question. An important part of this method involves computing a combined effect size across all of the studies. As such, this statistical approach involves extracting effect sizes and variance measures from various studies. By combining these effect sizes the statistical power is improved and can resolve uncertainties or discrepancies found in individual studies. Meta-analyses are integral in supporting research grant proposals, shaping treatment guidelines, and influencing health policies. They are also pivotal in summarizing existing research to guide future studies, thereby cementing their role as a fundamental methodology in metascience. Meta-analyses are often, but not always, important components of a systematic review.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures, diets or other medical treatments.
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints.
Reboxetine, sold under the brand name Edronax among others, is a drug of the norepinephrine reuptake inhibitor (NRI) class, marketed as an antidepressant by Pfizer for use in the treatment of major depression, although it has also been used off-label for panic disorder and attention deficit hyperactivity disorder (ADHD). It is approved for use in many countries worldwide, but has not been approved for use in the United States. Although its effectiveness as an antidepressant has been challenged in multiple published reports, its popularity has continued to increase.

Data dredging is the misuse of data analysis to find patterns in data that can be presented as statistically significant, thus dramatically increasing and understating the risk of false positives. This is done by performing many statistical tests on the data and only reporting those that come back with significant results.
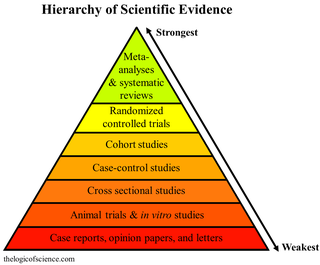
A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.
In statistics, sequential analysis or sequential hypothesis testing is statistical analysis where the sample size is not fixed in advance. Instead data is evaluated as it is collected, and further sampling is stopped in accordance with a pre-defined stopping rule as soon as significant results are observed. Thus a conclusion may sometimes be reached at a much earlier stage than would be possible with more classical hypothesis testing or estimation, at consequently lower financial and/or human cost.
In statistics, (between-) study heterogeneity is a phenomenon that commonly occurs when attempting to undertake a meta-analysis. In a simplistic scenario, studies whose results are to be combined in the meta-analysis would all be undertaken in the same way and to the same experimental protocols. Differences between outcomes would only be due to measurement error. Study heterogeneity denotes the variability in outcomes that goes beyond what would be expected due to measurement error alone.
In medicine an intention-to-treat (ITT) analysis of the results of a randomized controlled trial is based on the initial treatment assignment and not on the treatment eventually received. ITT analysis is intended to avoid various misleading artifacts that can arise in intervention research such as non-random attrition of participants from the study or crossover. ITT is also simpler than other forms of study design and analysis, because it does not require observation of compliance status for units assigned to different treatments or incorporation of compliance into the analysis. Although ITT analysis is widely employed in published clinical trials, it can be incorrectly described and there are some issues with its application. Furthermore, there is no consensus on how to carry out an ITT analysis in the presence of missing outcome data.
In epidemiology, reporting bias is defined as "selective revealing or suppression of information" by subjects. In artificial intelligence research, the term reporting bias is used to refer to people's tendency to under-report all the information available.

A forest plot, also known as a blobbogram, is a graphical display of estimated results from a number of scientific studies addressing the same question, along with the overall results. It was developed for use in medical research as a means of graphically representing a meta-analysis of the results of randomized controlled trials. In the last twenty years, similar meta-analytical techniques have been applied in observational studies and forest plots are often used in presenting the results of such studies also.
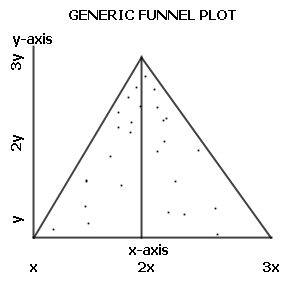
A funnel plot is a graph designed to check for the existence of publication bias; funnel plots are commonly used in systematic reviews and meta-analyses. In the absence of publication bias, it assumes that studies with high precision will be plotted near the average, and studies with low precision will be spread evenly on both sides of the average, creating a roughly funnel-shaped distribution. Deviation from this shape can indicate publication bias.

John P. A. Ioannidis is a Greek-American physician-scientist, writer and Stanford University professor who has made contributions to evidence-based medicine, epidemiology, and clinical research. Ioannidis studies scientific research itself - in other words, meta-research - primarily in clinical medicine and the social sciences.
In science, a null result is a result without the expected content: that is, the proposed result is absent. It is an experimental outcome which does not show an otherwise expected effect. This does not imply a result of zero or nothing, simply a result that does not support the hypothesis.
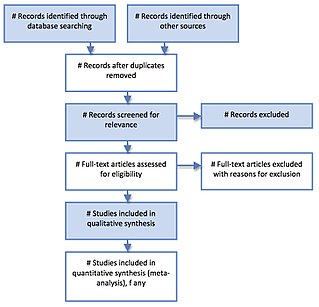
PRISMA is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research. The PRISMA standard superseded the earlier QUOROM standard. It offers the replicability of a systematic literature review. Researchers have to figure out research objectives that answer the research question, states the keywords, a set of exclusion and inclusion criteria. In the review stage, relevant articles were searched, irrelevant ones are removed. Articles are analyzed according to some pre-defined categories.
Estimation statistics, or simply estimation, is a data analysis framework that uses a combination of effect sizes, confidence intervals, precision planning, and meta-analysis to plan experiments, analyze data and interpret results. It complements hypothesis testing approaches such as null hypothesis significance testing (NHST), by going beyond the question is an effect present or not, and provides information about how large an effect is. Estimation statistics is sometimes referred to as the new statistics.

The replication crisis is an ongoing methodological crisis in which the results of many scientific studies are difficult or impossible to reproduce. Because the reproducibility of empirical results is an essential part of the scientific method, such failures undermine the credibility of theories building on them and potentially call into question substantial parts of scientific knowledge.
Preregistration is the practice of registering the hypotheses, methods, or analyses of a scientific study before it is conducted. Clinical trial registration is similar, although it may not require the registration of a study's analysis protocol. Finally, registered reports include the peer review and in principle acceptance of a study protocol prior to data collection.
Outcome switching is the practice of changing the primary or secondary outcomes of a clinical trial after its initiation. An outcome is the goal of the clinical trial, such as survival after five years for cancer treatment. Outcome switching can lead to bias and undermine the reliability of the trial, for instance when outcomes are switched after researchers already have access to trial data. That way, researchers can cherry pick an outcome which is statistically significant.











