Colloquially known as "downers", depressants or central nervous system (CNS) depressants are drugs that lower neurotransmission levels, decrease the electrical activity of brain cells, or reduce arousal or stimulation in various areas of the brain. Some specific depressants do influence mood, either positively or negatively, but depressants often have no clear impact on mood. In contrast, stimulants, or "uppers", increase mental alertness, making stimulants the opposite drug class from depressants. Antidepressants are defined by their effect on mood, not on general brain activity, so they form an orthogonal category of drugs.

Bicuculline is a phthalide-isoquinoline compound that is a light-sensitive competitive antagonist of GABAA receptors. It was originally identified in 1932 in plant alkaloid extracts and has been isolated from Dicentra cucullaria, Adlumia fungosa, and several Corydalis species. Since it blocks the inhibitory action of GABA receptors, the action of bicuculline mimics epilepsy; it also causes convulsions. This property is utilized in laboratories around the world in the in vitro study of epilepsy, generally in hippocampal or cortical neurons in prepared brain slices from rodents. This compound is also routinely used to isolate glutamatergic receptor function.
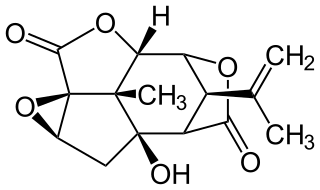
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison). A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Accurate regulation of GABAergic transmission through appropriate developmental processes, specificity to neural cell types, and responsiveness to activity is crucial for the proper functioning of nearly all aspects of the central nervous system (CNS). Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions and, to a lesser extent, bicarbonate ions.
Neuropharmacology is the study of how drugs affect function in the nervous system, and the neural mechanisms through which they influence behavior. There are two main branches of neuropharmacology: behavioral and molecular. Behavioral neuropharmacology focuses on the study of how drugs affect human behavior (neuropsychopharmacology), including the study of how drug dependence and addiction affect the human brain. Molecular neuropharmacology involves the study of neurons and their neurochemical interactions, with the overall goal of developing drugs that have beneficial effects on neurological function. Both of these fields are closely connected, since both are concerned with the interactions of neurotransmitters, neuropeptides, neurohormones, neuromodulators, enzymes, second messengers, co-transporters, ion channels, and receptor proteins in the central and peripheral nervous systems. Studying these interactions, researchers are developing drugs to treat many different neurological disorders, including pain, neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease, psychological disorders, addiction, and many others.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.
Cross-tolerance is a phenomenon that occurs when tolerance to the effects of a certain drug produces tolerance to another drug. It often happens between two drugs with similar functions or effects—for example, acting on the same cell receptor or affecting the transmission of certain neurotransmitters. Cross-tolerance has been observed with pharmaceutical drugs such as anti-anxiety agents and illicit substances, and sometimes the two of them together. Often, a person who uses one drug can be tolerant to a drug that has a completely different function. This phenomenon allows one to become tolerant to a drug that they have never used before.
An analeptic, in medicine, is a central nervous system stimulant. The term "analeptic" typically refers to respiratory stimulants. Analeptics are central nervous system (CNS) stimulants that include a wide variety of medications used to treat depression, attention deficit hyperactivity disorder (ADHD), and respiratory depression. Analeptics can also be used as convulsants, with low doses causing patients to experience heightened awareness, restlessness, and rapid breathing. The primary medical use of these drugs is as an anesthetic recovery tool or to treat emergency respiratory depression. Other drugs of this category are prethcamide, pentylenetetrazole, and nikethamide. Nikethamide is now withdrawn due to risk of convulsions. Analeptics have recently been used to better understand the treatment of a barbiturate overdose. Through the use of agents, researchers were able to treat obtundation and respiratory depression.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.
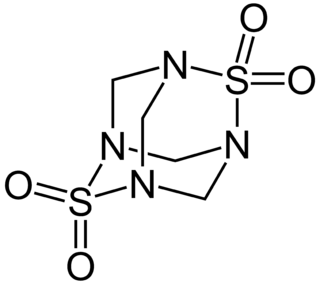
Tetramethylenedisulfotetramine (TETS) is an organic compound used as a rodenticide. It is an odorless, tasteless white powder that is slightly soluble in water, DMSO and acetone, and insoluble in methanol and ethanol. It is a sulfamide derivative. It can be synthesized by reacting sulfamide with formaldehyde solution in acidified water. When crystallized from acetone, it forms cubic crystals with a melting point of 255–260 °C.
GABA receptor antagonists are drugs that inhibit the action of GABA. In general these drugs produce stimulant and convulsant effects, and are mainly used for counteracting overdoses of sedative drugs.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce sedative effects. Furthermore, they lead to changes in gene transcription.
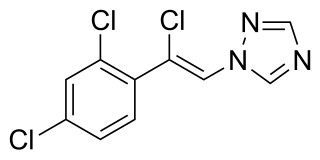
Loreclezole is a sedative and an anticonvulsant which acts as a GABAA receptor positive allosteric modulator. The binding site of loreclezole has been shown experimentally to be shared by valerenic acid, an extract of the root of the valerian plant. Structurally, loreclezole is a triazole derivative. In animal seizure models, loreclezole is protective against pentylenetetrazol seizures but is less active in the maximal electroshock test. In addition, at low, nontoxic doses, the drug has anti-absence activity in a genetic model of generalized absence epilepsy. Consequently, loreclezole has a profile of activity similar to that of benzodiazepines. A potential benzodiazepine-like interaction with GABA receptors is suggested by the observation that the anticonvulsant effects of loreclezole can be reversed by benzodiazepine receptor inverse agonists. The benzodiazepine antagonist flumazenil, however, fails to alter the anticonvulsant activity of loreclezole, indicating that loreclezole is not a benzodiazepine receptor agonist. Using native rat and cloned human GABA-A receptors, loreclezole strongly potentiated GABA-activated chloride current. However, activity of the drug did not require the presence of the γ-subunit and was not blocked by flumazenil, confirming that loreclezole does not interact with the benzodiazepine recognition site.

Ganaxolone, sold under the brand name Ztalmy, is a medication used to treat seizures in people with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder. Ganaxolone is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator.
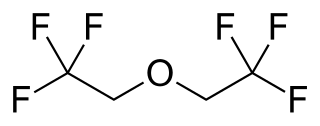
Flurothyl (Indoklon) is a volatile liquid drug from the halogenated ether family, related to inhaled anaesthetic agents such as diethyl ether, but having the opposite effects, acting as a stimulant and convulsant. A clear and stable liquid, it has a mild ethereal odor whose vapors are non-flammable. It is excreted from the body by the lungs in an unchanged state.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.
A channel modulator, or ion channel modulator, is a type of drug which modulates ion channels. They include channel blockers and channel openers.
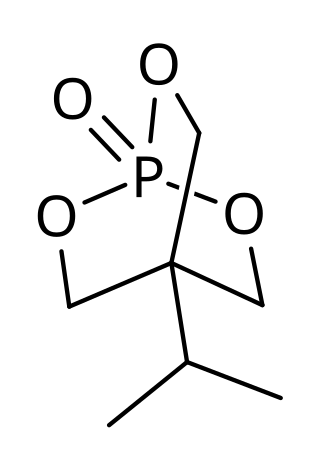
IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.
A GABAA receptor negative allosteric modulator is a negative allosteric modulator (NAM), or inhibitor, of the GABAA receptor, a ligand-gated ion channel of the major inhibitory neurotransmitter γ-aminobutyric acid (GABA). They are closely related and similar to GABAA receptor antagonists. The effects of GABAA receptor NAMs are functionally the opposite of those of GABAA receptor positive allosteric modulators (PAMs) like the benzodiazepines, barbiturates, and ethanol (alcohol). Non-selective GABAA receptor NAMs can produce a variety of effects including convulsions, neurotoxicity, and anxiety, among others.













