In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.
Ruthenium compounds are compounds containing the element ruthenium (Ru). Ruthenium compounds can have oxidation states ranging from 0 to +8, and −2. The properties of ruthenium and osmium compounds are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically.
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5.
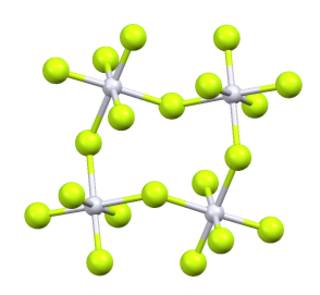
Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6 or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.
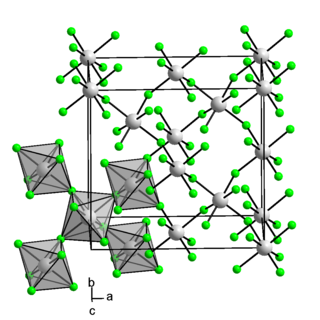
Iridium(IV) fluoride is a chemical compound of iridium and fluorine, with the chemical formula IrF4 and is a dark brown solid. Early reports of IrF4 prior to 1965 are questionable and appear to describe the compound IrF5. The solid can be prepared by reduction of IrF5 with iridium black or reduction with H2 in aqueous HF. The crystal structure of the solid is notable as it was the first example of a three-dimensional lattice structure found for a metal tetrafluoride and subsequently RhF4, PdF4 and PtF4 have been found to have the same structure. The structure has 6 coordinate, octahedral, iridium where two edges of the octahedra are shared and the two unshared fluorine atoms are cis to one another.
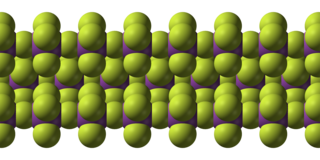
Bismuth pentafluoride is an inorganic compound with the formula BiF5. It is a white solid that is highly reactive. The compound is of interest to researchers but not of particular value.
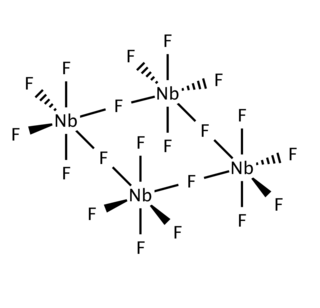
Niobium(V) fluoride, also known as niobium pentafluoride, is the inorganic compound with the formula NbF5. It is a colorless solid.
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionating to elemental iodine and iodine pentafluoride:
Pentafluoride may refer to:
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
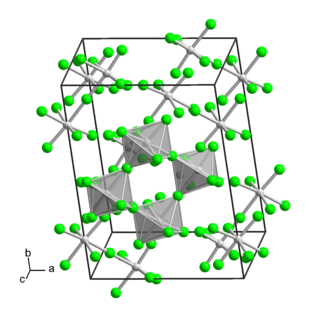
Molybdenum(V) fluoride is an inorganic compound with the formula MoF5. It is a hygroscopic yellow solid. Like most pentafluorides, it exists as a tetramer.

Ruthenium pentafluoride is the inorganic compound with the empirical formula RuF5. This green volatile solid has rarely been studied but is of interest as a binary fluoride of ruthenium, i.e. a compound containing only Ru and F. It is sensitive toward hydrolysis. Its structure consists of Ru4F20 tetramers, as seen in the isostructural platinum pentafluoride. Within the tetramers, each Ru adopts octahedral molecular geometry, with two bridging fluoride ligands.
Neptunium(V) fluoride or neptunium pentafluoride is a chemical compound of neptunium and fluorine with the formula NpF5.

Rhodium pentafluoride is an inorganic compound with the formula Rh4F20. It is a red solid. It is prepared by fluorination of rhodium trifluoride at 400 °C.
Osmium fluoride may refer to:







