The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.

Moderna, Inc. is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts, that focuses on RNA therapeutics, primarily mRNA vaccines. These vaccines use a copy of a molecule called messenger RNA (mRNA) to carry instructions for proteins to produce an immune response. The company's name is derived from the terms "modified", "RNA", and "modern".

Novavax, Inc. is an American biotechnology company based in Gaithersburg, Maryland, that develops vaccines to counter serious infectious diseases. Prior to 2020, company scientists developed experimental vaccines for influenza and respiratory syncytial virus (RSV), as well as Ebola and other emerging infectious diseases. During 2020, the company redirected its efforts to focus on development and approval of its NVX-CoV2373 vaccine for COVID-19.

An mRNAvaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules of antigen-encoding mRNA into cells, which use the designed mRNA as a blueprint to build foreign protein that would normally be produced by a pathogen or by a cancer cell. These protein molecules stimulate an adaptive immune response that teaches the body to identify and destroy the corresponding pathogen or cancer cells. The mRNA is delivered by a co-formulation of the RNA encapsulated in lipid nanoparticles that protect the RNA strands and help their absorption into the cells.
A respiratory syncytial virus vaccine, or RSV vaccine, is a vaccine that protects against respiratory syncytial virus. RSV affects an estimated 64 million people and causes 160,000 deaths worldwide each year.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).

Operation Warp Speed (OWS) was a public–private partnership initiated by the United States government to facilitate and accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. The first news report of Operation Warp Speed was on April 29, 2020, and the program was officially announced on May 15, 2020. It was headed by Moncef Slaoui from May 2020 to January 2021 and by David A. Kessler from January to February 2021. At the end of February 2021, Operation Warp Speed was transferred into the responsibilities of the White House COVID-19 Response Team.

Jason S. McLellan is a structural biologist, professor in the Department of Molecular Biosciences and Robert A. Welch Chair in Chemistry at The University of Texas at Austin who specializes in understanding the structure and function of viral proteins, including those of coronaviruses. His research focuses on applying structural information to the rational design of vaccines and other therapies for viruses, including SARS-CoV-2, the novel coronavirus that causes COVID-19, and respiratory syncytial virus (RSV). McLellan and his team collaborated with researchers at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center to design a stabilized version of the SARS-CoV-2 spike protein, which biotechnology company Moderna used as the basis for the vaccine mRNA-1273, the first COVID-19 vaccine candidate to enter phase I clinical trials in the U.S. At least three other vaccines use this modified spike protein: those from Pfizer and BioNTech; Johnson & Johnson and Janssen Pharmaceuticals; and Novavax.

BioNTech SE is a German biotechnology company based in Mainz that develops and manufactures active immunotherapies for patient-specific approaches to the treatment of diseases. It develops pharmaceutical candidates based on messenger ribonucleic acid (mRNA) for use as individualized cancer immunotherapies, as vaccines against infectious diseases and as protein replacement therapies for rare diseases, and also engineered cell therapy, novel antibodies and small molecule immunomodulators as treatment options for cancer.

The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech. For its development, BioNTech collaborated with the American company Pfizer to carry out clinical trials, logistics, and manufacturing. It is authorized for use in humans to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It is composed of nucleoside-modified mRNA (modRNA) that encodes a mutated form of the full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. Initial guidance recommended a two-dose regimen, given 21 days apart; this interval was subsequently extended to up to 42 days in the United States, and up to four months in Canada.
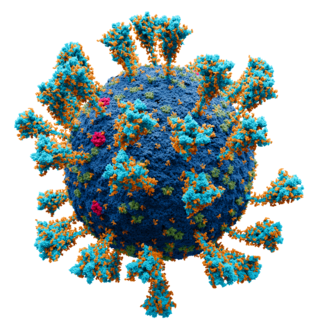
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations. It contains a recombinant spike protein from the SARS-CoV-2 Omicron variant lineage JN.1.
The COVID-19 vaccination program in the Philippines was a mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the pandemic in the country. The vaccination program was initiated by the Duterte administration on March 1, 2021, a day after the arrival of the country's first vaccine doses which were donated by the Chinese government.

The Janssen COVID‑19 vaccine, (Ad26.COV2.S) sold under the brand name Jcovden, is a COVID‑19 vaccine that was developed by Janssen Vaccines in Leiden, Netherlands, and its Belgian parent company Janssen Pharmaceuticals, a subsidiary of American company Johnson & Johnson.

SARS-CoV-2, the virus that causes COVID-19, was isolated in late 2019. Its genetic sequence was published on 11 January 2020, triggering an urgent international response to prepare for an outbreak and hasten the development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. By June 2020, tens of billions of dollars were invested by corporations, governments, international health organizations, and university research groups to develop dozens of vaccine candidates and prepare for global vaccination programs to immunize against COVID‑19 infection. According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.

COVID-19 vaccination in Canada is an ongoing, intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic in Canada. Provinces have worked with local municipal governments, hospital systems, family doctors and independently owned pharmacies to aid in part, or in full with vaccination rollout. The vaccination effort in full is the largest such immunization effort in the nation's history. The vaccination effort began December 14, 2020, and is currently ongoing.

The COVID-19 vaccination campaign in the United States is an ongoing mass immunization campaign for the COVID-19 pandemic in the United States. The Food and Drug Administration (FDA) first granted emergency use authorization to the Pfizer–BioNTech vaccine on December 10, 2020, and mass vaccinations began four days later. The Moderna vaccine was granted emergency use authorization on December 17, 2020, and the Janssen vaccine was granted emergency use authorization on February 27, 2021. It was not until April 19, 2021, that all U.S. states had opened vaccine eligibility to residents aged 16 and over. On May 10, 2021, the FDA approved the Pfizer-BioNTech vaccine for adolescents aged 12 to 15. On August 23, 2021, the FDA granted full approval to the Pfizer–BioNTech vaccine for individuals aged 16 and over.

The Sanofi–GSK COVID-19 vaccine sold under the brand name VidPrevtyn Beta, is a COVID-19 vaccine developed by Sanofi Pasteur and GSK.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness, and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:

In many countries, the dissemination of varied claims and perspectives regarding COVID-19 vaccines has sparked widespread public discussion. These include concerns about potential side effects, differing interpretations of how the immune system responds to vaccination, and debates over the development and distribution of COVID-19 vaccines. Additionally, stories such as COVID-19 being linked to 5G technology and other debated information have also emerged. This spread of information, including content from anti-vaccination advocates, may have influenced people's attitudes towards vaccination. In response, governments and private organizations around the world have introduced measures to encourage or mandate vaccination, such as lotteries, mandates, and free entry to events. These measures have further fueled debates about their legality and effectiveness.




!["Moderna arm", a non-serious skin reaction rarely seen [?]7 days after injection with the Moderna vaccine. ModernaReaction.jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b7/ModernaReaction.jpg/170px-ModernaReaction.jpg)

















