Related Research Articles

Gluten is a structural protein naturally found in certain cereal grains. The term gluten usually refers to the elastic network of a wheat grain's proteins, gliadin and glutenin primarily, that forms readily with the addition of water and often kneading in the case of bread dough. The types of grains that contain gluten include all species of wheat, and barley, rye, and some cultivars of oat; moreover, cross hybrids of any of these cereal grains also contain gluten, e.g. triticale. Gluten makes up 75–85% of the total protein in bread wheat.

Coeliac disease or celiac disease is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barley. Classic symptoms include gastrointestinal problems such as chronic diarrhoea, abdominal distention, malabsorption, loss of appetite, and among children failure to grow normally. Non-classic symptoms are more common, especially in people older than two years. There may be mild or absent gastrointestinal symptoms, a wide number of symptoms involving any part of the body, or no obvious symptoms. Coeliac disease was first described in childhood; however, it may develop at any age. It is associated with other autoimmune diseases, such as Type 1 diabetes mellitus and Hashimoto's thyroiditis, among others.
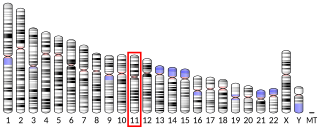
Secretin is a hormone that regulates water homeostasis throughout the body and influences the environment of the duodenum by regulating secretions in the stomach, pancreas, and liver. It is a peptide hormone produced in the S cells of the duodenum, which are located in the intestinal glands. In humans, the secretin peptide is encoded by the SCT gene.

A gluten-free diet (GFD) is a nutritional plan that strictly excludes gluten, which is a mixture of prolamin proteins found in wheat, as well as barley, rye, and oats. The inclusion of oats in a gluten-free diet remains controversial, and may depend on the oat cultivar and the frequent cross-contamination with other gluten-containing cereals.

Casomorphin is an opioid peptide derived from the digestion of the milk protein casein.

An enkephalin is a pentapeptide involved in regulating nociception in the body. The enkephalins are termed endogenous ligands, as they are internally derived and bind as ligands to the body's opioid receptors. Discovered in 1975, two forms of enkephalin have been found, one containing leucine ("leu"), and the other containing methionine ("met"). Both are products of the proenkephalin gene.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Gliadin is a class of proteins present in wheat and several other cereals within the grass genus Triticum. Gliadins, which are a component of gluten, are essential for giving bread the ability to rise properly during baking. Gliadins and glutenins are the two main components of the gluten fraction of the wheat seed. This gluten is found in products such as wheat flour. Gluten is split about evenly between the gliadins and glutenins, although there are variations found in different sources.

Opioid peptides or opiate peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, control of food intake, and the rewarding effects of alcohol and nicotine.
The rubiscolins are a group of opioid peptides that are formed during digestion of the ribulose bisphosphate carboxylase/oxygenase (Rubisco) protein from spinach leaves. These peptides have much in common with the better-known gluten exorphins.
Intestinal permeability is a term describing the control of material passing from inside the gastrointestinal tract through the cells lining the gut wall, into the rest of the body. The intestine normally exhibits some permeability, which allows nutrients to pass through the gut, while also maintaining a barrier function to keep potentially harmful substances from leaving the intestine and migrating to the body more widely. In a healthy human intestine, small particles can migrate through tight junction claudin pore pathways, and particles up to 10–15 Å can transit through the paracellular space uptake route. There is some evidence abnormally increased intestinal permeability may play a role in some chronic diseases and inflammatory conditions. The most well understood condition with observed increased intestinal permeability is celiac disease.
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds.

Gluten-related disorders is the term for the diseases triggered by gluten, including celiac disease (CD), non-celiac gluten sensitivity (NCGS), gluten ataxia, dermatitis herpetiformis (DH) and wheat allergy. The umbrella category has also been referred to as gluten intolerance, though a multi-disciplinary physician-led study, based in part on the 2011 International Coeliac Disease Symposium, concluded that the use of this term should be avoided due to a lack of specificity.
Leu-enkephalin is an endogenous opioid peptide neurotransmitter with the amino acid sequence Tyr-Gly-Gly-Phe-Leu that is found naturally in the brains of many animals, including humans. It is one of the two forms of enkephalin; the other is met-enkephalin. The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine. Leu-enkephalin has agonistic actions at both the μ- and δ-opioid receptors, with significantly greater preference for the latter. It has little to no effect on the κ-opioid receptor.
Big dynorphin is an endogenous opioid peptide of the dynorphin family that is composed of both dynorphin A and dynorphin B. Big dynorphin has the amino acid sequence: Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln-Lys-Arg-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr. It has nociceptive and anxiolytic-like properties, as well as effects on memory in mice.
The immunochemistry of Triticeae glutens is important in several inflammatory diseases. It can be subdivided into innate responses, class II mediated presentation, class I mediated stimulation of killer cells, and antibody recognition. The responses to gluten proteins and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen genes. In gluten sensitive enteropathy, there are four types of recognition, innate immunity, HLA-DQ, and antibody recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.
Exorphins are exogenous opioid peptides, distinguished from endorphins, or endogenous opioid peptides.
Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the other deltorphins (such as deltorphin I and deltorphin II) and the dermorphins, deltorphin is endogenous to frogs of the genus Phyllomedusa such as P. bicolor and P. sauvagei where it is produced in their skin, and is not known to occur naturally in any other species. Deltorphin is one of the highest affinity and most selective naturally occurring opioid peptides known, acting as a very potent and highly specific agonist of the δ-opioid receptor.
The opioid excess theory is a theory which postulates that autism is the result of a metabolic disorder in which opioid peptides produced through metabolism of gluten and casein pass through an abnormally permeable intestinal membrane and then proceed to exert an effect on neurotransmission through binding with opioid receptors. It is believed by advocates of this hypothesis that autistic children are unusually sensitive to gluten, which results in small bowel inflammation in these children, which in turn allows these opioid peptides to enter the brain.
Non-celiac gluten sensitivity (NCGS) or gluten sensitivity is a controversial disorder which can cause both gastrointestinal and other problems.
References
- ↑ Morley JE, Levine AS, Yamada T, Gebhard RL, Prigge WF, Shafer RB, et al. (June 1983). "Effect of exorphins on gastrointestinal function, hormonal release, and appetite". Gastroenterology. 84 (6): 1517–23. doi: 10.1016/0016-5085(83)90374-8 . PMID 6840480.
- 1 2 Pruimboom L, de Punder K (November 2015). "The opioid effects of gluten exorphins: asymptomatic celiac disease". Journal of Health, Population, and Nutrition. 33 (1): 24. doi: 10.1186/s41043-015-0032-y . PMC 5025969 . PMID 26825414.
- ↑ Trivedi MS, Shah JS, Al-Mughairy S, Hodgson NW, Simms B, Trooskens GA, et al. (October 2014). "Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences". The Journal of Nutritional Biochemistry. 25 (10): 1011–8. doi:10.1016/j.jnutbio.2014.05.004. PMC 4157943 . PMID 25018147.
- ↑ Whiteley P, Shattock P, Knivsberg AM, Seim A, Reichelt KL, Todd L, et al. (2013). "Gluten- and casein-free dietary intervention for autism spectrum conditions". Frontiers in Human Neuroscience. 6: 344. doi: 10.3389/fnhum.2012.00344 . PMC 3540005 . PMID 23316152.