
γ-Aminobutyric acid, or GABA, is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.

The GABA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), the chief inhibitory compound in the mature vertebrate central nervous system. There are two classes of GABA receptors: GABAA and GABAB. GABAA receptors are ligand-gated ion channels ; whereas GABAB receptors are G protein-coupled receptors, also called metabotropic receptors.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.
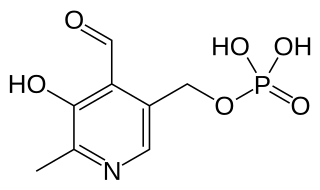
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase, is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.
Malate dehydrogenase (EC 1.1.1.37) (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate dehydrogenases, which have other EC numbers and catalyze other reactions oxidizing malate, have qualified names like malate dehydrogenase (NADP+).
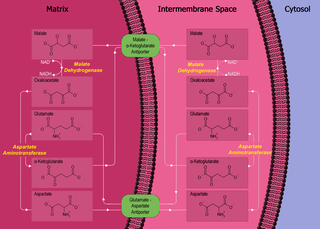
The malate–aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryotes. These electrons enter the electron transport chain of the mitochondria via reduction equivalents to generate ATP. The shuttle system is required because the mitochondrial inner membrane is impermeable to NADH, the primary reducing equivalent of the electron transport chain. To circumvent this, malate carries the reducing equivalents across the membrane.
Branched-chain amino acid aminotransferase (BCAT), also known as branched-chain amino acid transaminase, is an aminotransferase enzyme (EC 2.6.1.42) which acts upon branched-chain amino acids (BCAAs). It is encoded by the BCAT2 gene in humans. The BCAT enzyme catalyzes the conversion of BCAAs and α-ketoglutarate into branched chain α-keto acids and glutamate.

γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), and sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan, and Mexico. It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.
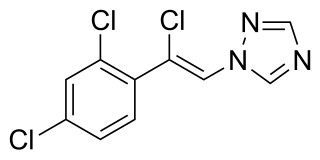
Loreclezole is a sedative and an anticonvulsant which acts as a GABAA receptor positive allosteric modulator. The binding site of loreclezole has been shown experimentally to be shared by valerenic acid, an extract of the root of the valerian plant. Structurally, loreclezole is a triazole derivative. In animal seizure models, loreclezole is protective against pentylenetetrazol seizures but is less active in the maximal electroshock test. In addition, at low, nontoxic doses, the drug has anti-absence activity in a genetic model of generalized absence epilepsy. Consequently, loreclezole has a profile of activity similar to that of benzodiazepines. A potential benzodiazepine-like interaction with GABA receptors is suggested by the observation that the anticonvulsant effects of loreclezole can be reversed by benzodiazepine receptor inverse agonists. The benzodiazepine antagonist flumazenil, however, fails to alter the anticonvulsant activity of loreclezole, indicating that loreclezole is not a benzodiazepine receptor agonist. Using native rat and cloned human GABA-A receptors, loreclezole strongly potentiated GABA-activated chloride current. However, activity of the drug did not require the presence of the γ-subunit and was not blocked by flumazenil, confirming that loreclezole does not interact with the benzodiazepine recognition site.
In enzymology, 4-aminobutyrate transaminase, also called GABA transaminase or 4-aminobutyrate aminotransferase, or GABA-T, is an enzyme that catalyzes the chemical reaction:
Aspartate aminotransferase, mitochondrial is an enzyme that in humans is encoded by the GOT2 gene. Glutamic-oxaloacetic transaminase is a pyridoxal phosphate-dependent enzyme which exists in cytoplasmic and inner-membrane mitochondrial forms, GOT1 and GOT2, respectively. GOT plays a role in amino acid metabolism and the urea and Kreb's cycle. Also, GOT2 is a major participant in the malate-aspartate shuttle, which is a passage from the cytosol to the mitochondria. The two enzymes are homodimeric and show close homology. GOT2 has been seen to have a role in cell proliferation, especially in terms of tumor growth.

Malate dehydrogenase, mitochondrial also known as malate dehydrogenase 2 is an enzyme that in humans is encoded by the MDH2 gene.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Gabaculine is a naturally occurring neurotoxin first isolated from the bacteria Streptomyces toyacaensis, which acts as a potent and irreversible GABA transaminase inhibitor, and also a GABA reuptake inhibitor. Gabaculine is also known as 3-amino-2,3-dihydrobenzoic acid hydrochloride and 5-amino cyclohexa-1,3 dienyl carboxylic acid. Gabaculine increased GABA levels in the brain and had an effect on convulsivity in mice.

A GABA reuptake inhibitor (GRI) is a type of drug which acts as a reuptake inhibitor for the neurotransmitter gamma-Aminobutyric acid (GABA) by blocking the action of the gamma-Aminobutyric acid transporters (GATs). This in turn leads to increased extracellular concentrations of GABA and therefore an increase in GABAergic neurotransmission. Gamma-aminobutyric acid (GABA) is an amino acid that functions as the predominant inhibitory neurotransmitter within the central nervous system, playing a crucial role in modulating neuronal activity in both the brain and spinal cord. While GABA predominantly exerts inhibitory actions in the adult brain, it has an excitatory role during developmental stages. When the neuron receives the action potential, GABA is released from the pre-synaptic cell to the synaptic cleft. After the action potential transmission, GABA is detected on the dendritic side, where specific receptors collectively contribute to the inhibitory outcome by facilitating GABA transmitter uptake. Facilitated by specific enzymes, GABA binds to post-synaptic receptors, with GABAergic neurons playing a key role in system regulation. The inhibitory effects of GABA diminish when presynaptic neurons reabsorb it from the synaptic cleft for recycling by GABA transporters (GATs). The reuptake mechanism is crucial for maintaining neurotransmitter levels and synaptic functioning. Gamma-aminobutyric acid Reuptake Inhibitors (GRIs) hinder the functioning of GATs, preventing GABA reabsorption in the pre-synaptic cell. This results in increased GABA levels in the extracellular environment, leading to elevated GABA-mediated synaptic activity in the brain.

Cartazolate (SQ-65,396) is a drug of the pyrazolopyridine class. It acts as a GABAA receptor positive allosteric modulator at the barbiturate binding site of the complex and has anxiolytic effects in animals. It is also known to act as an adenosine antagonist at the A1 and A2 subtypes and as a phosphodiesterase inhibitor. Cartazolate was tested in human clinical trials and was found to be efficacious for anxiety but was never marketed. It was developed by a team at E.R. Squibb and Sons in the 1970s.
4-aminobutyrate---pyruvate transaminase is an enzyme with systematic name 4-aminobutanoate:pyruvate aminotransferase. This enzyme is a type of GABA transaminase, which degrades the neurotransmitter GABA. The enzyme catalyses the following chemical reaction

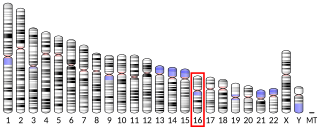
4-Aminobutyrate aminotransferase is a protein that in humans is encoded by the ABAT gene. This gene is located in chromosome 16 at position of 13.2. This gene goes by a number of names, including, GABA transaminase, GABAT, 4-aminobutyrate transaminase, NPD009 etc. This gene is mainly and abundant located in neuronal tissues. 4-Aminobutyrate aminotransferase belongs to group of pyridoxal 5-phosphate-dependent enzyme which activates a large portion giving reaction to amino acids. ABAT is made up of two monomers of enzymes where each subunit has a molecular weight of 50kDa. It is identified that almost tierce of human synapses have GABA. GABA is a neurotransmitter that has different roles in different regions of the central and peripheral nervous systems. It can be found also in some tissues that do not have neurons. In addition, GAD and GABA-AT are responsible in regulating the concentration of GABA.
Fernando Garcia de Mello is a renowned neurochemist from Brazil. He obtained his degree in Biochemistry in 1968 from the State University of Rio de Janeiro. Fernando Mello started his scientific training as an undergraduate student at the Brazilian National Institute of Cancer, and later at the Institute of Biophysics from the Federal University of Rio de Janeiro, being mentored by dr. Firmino de Castro, which greatly influenced him to have a more humanistic approach towards the students that he would train. It was only during his post-doc period (1973-1976) at the National Institutes of Health under supervision of dr. Marshall Warren Nirenberg that Mello began his research in Neurochemistry, using the embryonary Retina as a model for his investigations.

















