Volume 28, Number 11—November 2022
Research Letter
Monkeypox Virus Transmission to Healthcare Worker through Needlestick Injury, Brazil
Abstract
We describe monkeypox virus (MPXV) transmission from a patient to a healthcare worker through needlestick injury. A lesion appeared at the inoculation site 5 days after injury. Blood tested MPXV-positive by PCR before symptoms worsened; blood remained MPXV-positive at discharge 19 days after symptom onset. Postexposure prophylaxis could prevent potential MPXV bloodborne transmission.
In July 2022, the World Health Organization declared the global monkeypox outbreak a public health emergency (1). Monkeypox virus (MPXV) is transmitted through close or direct contact with skin lesions or respiratory droplets and through fomites, but knowledge gaps about transmission persist.
During the ongoing outbreak, MPX has disproportionately affected men who have sex with men, suggesting amplification through sexual networks (2). MPXV transmission to healthcare workers (HCWs) in endemic settings is well described (3) but has not been well characterized in the current outbreak. In nonendemic countries, monkeypox is rare, and standard infection control precautions are applied, suggesting HCWs are at low risk of acquiring MPXV; only 1 prior HCW case has been reported (4). We describe MPXV transmission to a HCW in Brazil through a needlestick injury.
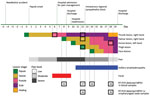
On July 9, 2022, a female nurse in her 20s sustained a needlestick injury to her thumb from supplies used to collect cutaneous lesion samples from a monkeypox patient. The nurse was wearing personal protective equipment, including gown, gloves, goggles, and mask, and was gathering materials to discard in a sharps container when a needle perforated her glove; the puncture site was visible immediately. After 5 days, a nodule developed at the injury site (day 0 of symptoms); it later evolved into a painful vesicle (Figure). The nurse lived alone, denied recent travel, and reported having protected sexual intercourse only with her male partner. She had no other potential exposures.
The source patient, a man in his 20s who reported having sex with men, had mild monkeypox that started 2 weeks before the needlestick incident. He had sore throat, cervical lymphadenopathy, and sparse lesions on his face, torso, and groin. The patient and nurse provided written consent for this report.
Overall, the nurse had 7 lesions: 1 each on the thumb (inoculation site) and palm of the right hand, dorsal left hand, and left thigh, and 3 on her face (Appendix Figures 1–3). Magnetic resonance imaging of the injury site on day 15 showed a neurovascular bundle and subcutaneous inflammation.
During the nurse’s follow-up, blood and skin lesion samples tested MPXV-positive by reverse transcription PCR using the QIAamp Viral DNA Mini Kit (QIAGEN, https://www.qiagen.com) for DNA extraction and TaqMan Monkeypox Virus Microbe Detection Assay (Thermo Fisher Scientific, https://www.thermofisher.com) for amplification. MPXV also was detectable in oropharyngeal samples despite the absence of respiratory symptoms. Of note, all collected specimens had detectable MPXV DNA throughout hospitalization. The nurse was discharged to outpatient care before complete lesion resolution (Figure).
In nonendemic settings, needlestick injury is an unusual form of patient-to-HCW MPXV transmission. Before 2022, fewer human-to-human than animal-to-human MPXV transmission cases were reported during outbreaks in Africa (5). In nonendemic countries, sporadic zoonotic or travel-associated monkeypox outbreaks have occurred (5,6), but during May–September 2022, >50,000 cases were reported worldwide (https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html), mainly through sexual or intimate contact transmission (7). HCWs are at risk, but a recent review of MPXV transmission in healthcare facilities in nonendemic countries found only 1 documented case of nosocomial monkeypox in a HCW, probably through contact with contaminated bedding (4,8).
Our case enabled observation of the natural progression of monkeypox through longitudinal clinical and laboratory monitoring of disease stages. The incubation period was 5 days. A cutaneous lesion and pain and inflammation at the inoculation site preceded generalized symptoms of fever and lymphadenopathy. The transmission route might have influenced the absence of a prodromal phase in the nurse because needlestick transmission parallels bite or scratch transmission from MPXV-infected animals to humans; in those cases, a febrile prodrome is uncommon (5). In addition, the nurse experienced severe injury site pain, which coincides with a series of cases in the current outbreak in which most patients who acquired MPXV by sexual or intimate contact were hospitalized for severe anorectal pain (2). The pain similarity suggests that the primary MPXV inoculation site is associated with painful lesions and possible neural impairment, as implied by the nurse’s magnetic resonance images.
MPXV DNA detected in the nurse’s blood on day 8, before skin lesions appeared at distant sites, suggests hematogenous virus dissemination. Few reports describe MPXV DNA in blood, but a retrospective study of monkeypox antiviral treatment found detectable MPXV DNA in blood after 14 days, even after skin lesions resolved (8). How detectable MPXV DNA corresponds to true viremia is unknown, but persistent DNA suggests bloodborne transmission could be possible through needlesticks, blood transfusions, and organ transplants. Persistent MPXV DNA in the nurse’s oropharyngeal samples aligns with another report (9), but efficiency for droplet or airborne transmission remains unknown.
Because few documented needlestick monkeypox cases are available (9), we could not estimate transmission risk, but instruments used on cutaneous lesions likely pose a high risk. The World Health Organization recommends postexposure prophylaxis with second- or third-generation vaccine, if available, up to 4 days after exposure (10). The state of São Paulo, Brazil, discontinued smallpox vaccination after 1979, and no smallpox or monkeypox vaccine is available in Brazil. However, HCWs should be considered for vaccination as soon as it is available.
Our report describes clinical features of monkeypox, including extreme pain at the inoculation site and prolonged DNAemia, after needlestick transmission in a HCW. Preexposure and postexposure prophylaxis, including vaccination, should be provided for HCWs in Brazil.
Dr. Bubach Carvalho is an infectious disease specialist at Universidade de São Paulo Hospital das Clínicas, São Paulo, Brazil. Her primary research interests include nosocomial disease transmission and hospital infection control procedures.
References
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022 [cited 2022 Aug 22]. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al.; SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–91. DOIPubMedGoogle Scholar
- Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13:
e0007791 . DOIPubMedGoogle Scholar - Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782–5. DOIPubMedGoogle Scholar
- Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–80. DOIPubMedGoogle Scholar
- Angelo KM, Petersen BW, Hamer DH, Schwartz E, Brunette G. Monkeypox transmission among international travellers-serious monkey business? J Travel Med. 2019;26:
taz002 . DOIPubMedGoogle Scholar - Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al.; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–62. DOIPubMedGoogle Scholar
- Zachary KC, Shenoy ES. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: Low risk but limited literature. Infect Control Hosp Epidemiol. 2022;43:920–4. DOIPubMedGoogle Scholar
- Loeb M, Zando I, Orvidas MC, Bialachowski A, Groves D, Mahoney J. Laboratory-acquired vaccinia infection. Can Commun Dis Rep. 2003;29:134–6.PubMedGoogle Scholar
- WHO Emergency Response Team. Vaccines and immunization for monkeypox: interim guidance, 14 June 2022 [cited 2022 Aug 22]. https://www.who.int/publications/i/item/who-mpx-immunization-2022.1
Figure
Cite This ArticleOriginal Publication Date: September 19, 2022
Table of Contents – Volume 28, Number 11—November 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hermes R. Higashino, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Divisão de Moléstias Infecciosas e Parasitárias, Ave. Dr. Enéas de Carvalho Aguiar, 255 – 4o andar, São Paulo 05403-000, Brazil
Top