Volume 21, Number 4—April 2015
Letter
Nairobi Sheep Disease Virus RNA in Ixodid Ticks, China, 2013
To the Editor: Nairobi sheep disease virus (NSDV; genus Nairovirus, family Bunyaviridae) causes acute hemorrhagic gastroenteritis in sheep and goats (1,2). First identified in Nairobi, Kenya, in 1910, it is considered endemic in East Africa (1,3). Ganjam virus, a variant of NSDV, is found in India and Sri Lanka (2). NSDV has a limited effect on animals bred in areas to which the virus is endemic but can cause large losses of animals during introduction of new livestock or transport of animals through these areas (4). In humans, NSDV infection can cause febrile illness, headache, nausea, and vomiting (5).
Ticks are the main transmission vectors of NSDV and many other viral pathogens and therefore pose a major threat to public health (6,7). Here, we describe a newly discovered NSDV, named NSDV (China), identified by viral metagenomic analysis of ticks collected from the northeast region of the People’s Republic of China (Liaoning, Jilin, and Heilongjiang provinces) during May–July, 2013, and divided into 9 groups according to tick species and sampling sites. Four tick species were morphologically identified: Haemaphysalis longicornis (84.8%); Dermacentor silvarum (7.2%); D. nuttalli (5.5%); and Ixodes persulcatus (2.5%) (Technical Appendix Table 1).
Of the 6,427 ticks collected, 3,410 were divided into 9 groups (average 379 ticks/group, range 163–512); each group was homogenized in SM buffer (50 mmol/L Tris, 10 mmol/L MgSO4, 0.1 mol/L NaCl, pH 7.5). Viral RNA extraction, Solexa sequencing, and analysis are described in the online Technical Appendix. Among the sequences annotated to mammalian viruses, 65 contigs were found to target the small (n = 15), medium (n = 27), and large (n = 23) segments of the NSDV genome (Technical Appendix Tables 2–4).
To confirm the Solexa results, a 376-nt fragment of the NSDV small gene segment was amplified by reverse transcription PCR (RT-PCR) by using primers P1 (5′-AGCAAAGAGCACATTGACTGGGC-3′) and P2 (5′-CTGTCACACCTGCCTTCCAA-3′). Ticks in 3 H. longicornis groups were positive for NSDV: group 1 from sheep in Jian, Jilin Province (125°34′E, 40°52′N); group 2 from cattle in Jinxing, Jilin Province (130°38′E, 42°25′N); and group 5 from sheep in Dandong, Liaoning Province (124°23′E, 40°07′N). Ticks in the other groups were negative. The obtained sequences shared 92% identity with NSDV from H. intermedia in India.
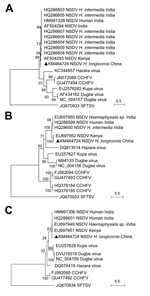
The full-length sequence of NSDV was then obtained from group 2 by RT-PCR by using primers based on the Solexa sequences or the conserved sequences of nairoviruses (Technical Appendix Table 5). The complete sequences of the small, medium, and large segments of NSDV (China) (GenBank accession nos. KM464724–KM464726) contained 1,590, 5,077, and 12,081 nt, respectively; that is, they were similar to other NSDVs. Sequence comparisons showed 75.1%–89.6% identity with other NSDVs at the nucleotide level and 81.3%–96.7% at the deduced amino acid level (Technical Appendix Table 6). Compared with other member species within the genus Nairovirus (Dugbe, Kupe, Hazara, and Crimean Congo hemorrhagic fever viruses), low identities (37.5%–68.6%) were observed at both nucleotide and amino acid levels (Technical Appendix Table 6). Phylogenetic analysis based on the amino acid sequences grouped the virus together with NSDVs from Africa and South Asia (Figure).
The remaining tick samples of the NSDV-positive groups were used to determine the infection frequency by using RT-PCR to analyze primers P1 and P2. We assayed 104 tick pools (average 15 ticks/pool, range 8–40), 13 pools of 416 ticks in Jian Province and 91 pools of 1,095 ticks in Jinxing Province; 12.5% (13/104) tested positive, 38.5% (5/13) in Jian and 8.8% (8/91) in Jinxing. The higher prevalence in Jian Province may result from more ticks in the pools. Attempts to isolate virus from the positive samples in cell lines (Vero and BHK-21) and suckling mice were unsuccessful; thus, its pathogenicity could not be determined.
In Africa, NSDV is primarily transmitted by R. appendiculatus ticks (5). In South Asia (India and Sri Lanka), NSDV has been isolated from ticks (H. intermedia, H. wellingtoni, and R. haemaphysaloides), mosquitoes, sheep and humans; H. intermedia ticks are considered the main vector for the virus (5,8,9). NSDV had not previously been reported from East Asia. The isolate we identified, NSDV (China), is genetically divergent from the NSDVs of South Asia and Africa and is therefore a novel strain, with H. longicornis likely the main vector. Nairobi sheep disease has not been reported in China and East Asia, but our results indicate the risk of its occurrence in these regions, where H. longicornis is widely distributed (10). More extensive investigation to clarifty the natural circulation of NSDV among ticks should be conducted and surveillance of sheep improved to prevent outbreaks of Nairobi sheep disease in China and East Asia.
Acknowledgment
This work was supported by the Science and Technology Basic Work Program from the Ministry of Science and Technology of China (2013FY113600), and the Military Medical Innovation Program of Academy of Military Medical Sciences (2012CXJJ019).
References
- Montgomery E. On a tick-borne gastro-enteritis of sheep and goats occurring in East Africa. J Comp Pathol Ther. 1917;30:28–57. DOIGoogle Scholar
- Marczinke BI, Nichol ST. Nairobi sheep disease virus, an important tick-borne pathogen of sheep and goats in Africa, is also present in Asia. Virology. 2002;303:146–51. DOIPubMedGoogle Scholar
- Weinbren MP, Gourlay RN, Lumsden WHR, Weinbren WM. An epizootic of Nairobi sheep disease in Uganda. J Comp Pathol Ther. 1958;68:174–87. DOIPubMedGoogle Scholar
- Lasecka L, Baron MD. The nairovirus Nairobi sheep disease virus/Ganjam virus induces the translocation of protein disulphide isomerase-like oxidoreductases from the endoplasmic reticulum to the cell surface and the extracellular space. PLoS ONE. 2014;9:e94656. DOIPubMedGoogle Scholar
- Yadav PD, Vincent MJ, Khristova M, Kale C, Nichol ST, Mishra AC, Genomic analysis reveals Nairobi sheep disease virus to be highly diverse and present in both Africa, and in India in the form of the Ganjam virus variant. Infect Genet Evol. 2011;11:1111–20. DOIPubMedGoogle Scholar
- Perera LP, Peiris JSM, Weilgama DJ, Calisher CH, Shope RE. Nairobi sheep disease virus isolated from Haemaphysalis intermedia ticks collected in Sri Lanka. Ann Trop Med Parasitol. 1996;90:91–3 .PubMedGoogle Scholar
- Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14:763–72. DOIPubMedGoogle Scholar
- Rajagopalan PK, Sreenivasan MA, Paul SD. Isolation of Ganjam virus from the bird tick Haemaphysalis wellingtoni Nuttall and Warburton 1907. Indian J Med Res. 1970;58:1195–6 .PubMedGoogle Scholar
- Joshi MV, Geevarghese G, Joshi GD, Ghodke YS, Mourya DT, Mishra AC. Isolation of Ganjam virus from ticks collected of domestic animals around Pune, Maharashtra, India. J Med Entomol. 2005;42:204–6. DOIPubMedGoogle Scholar
- Hoogstraal H, Roberts FH, Kohls GM, Tipton VJ. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J Parasitol. 1968;54:1197–213. DOIPubMedGoogle Scholar
Figure
Cite This Article1These authors contributed equally to this article.
Related Links
Table of Contents – Volume 21, Number 4—April 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Quan Liu, Key Laboratory of Jilin Province for Zoonosis Prevention and Control, Military Veterinary Institute, Academy of Military Medical Sciences, 666 Liuying West Rd, Jingyue Economic Development Zone, Changchun, 130122, People’s Republic of China
Top