
A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus with the empirical formula Cm(H2O)n, which does not mean the H has covalent bonds with O. However, not all carbohydrates conform to this precise stoichiometric definition, nor are all chemicals that do conform to this definition automatically classified as carbohydrates.

Cis–trans isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. Chemically, monosaccharides are polyhydroxy aldehydes with the formula H-[CHOH]
n-CHO or polyhydroxy ketones with the formula H-[CHOH]
m-CO-[CHOH]
n-H with three or more carbon atoms.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
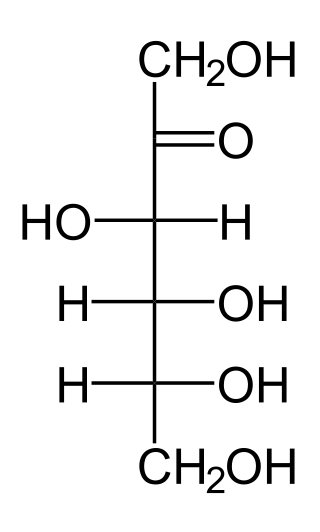
In chemistry, a hexose is a monosaccharide with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
In chemistry, a racemic mixture or racemate is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates.

The structural formula of a chemical compound is a graphic representation of the molecular structure, showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections.
An aldose is a monosaccharide with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ketoses, which have the carbonyl group away from the end of the molecule, and are therefore ketones.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
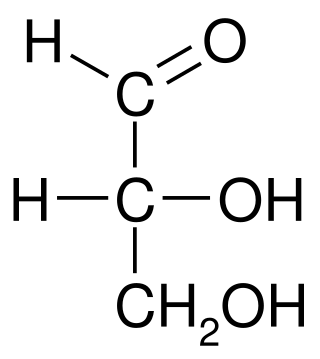
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers.
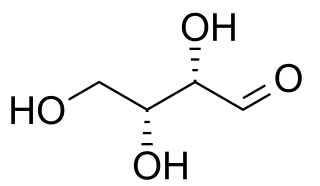
In stereochemistry, diastereomers are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.

A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is superposable on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.
Aldaric acids are a group of sugar acids, where the terminal hydroxyl and carbonyl groups of the sugars have been replaced by terminal carboxylic acids, and are characterised by the formula HO2C-(CHOH)n-CO2H. Oxidation of just the aldehyde yields an aldonic acid while oxidation of just the terminal hydroxyl group yields an uronic acid.) Aldaric acids cannot form cyclic hemiacetals like unoxidized sugars, but they can sometimes form lactones.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

Asymmetric induction describes the preferential formation in a chemical reaction of one enantiomer (enantioinduction) or diastereoisomer (diastereoinduction) over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.

In chemistry, absolute configuration refers to the spatial arrangement of atoms within a molecular entity that is chiral, and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules where carbon is bonded to four different substituents. This type of construction creates two possible enantiomers. Absolute configuration uses a set of rules to describe the relative positions of each bond around the chiral center atom. The most common labeling method uses the descriptors R or S and is based on the Cahn–Ingold–Prelog priority rules. R and S refer to rectus and sinister, Latin for right and left, respectively.
Monosaccharide nomenclature is the naming system of the building blocks of carbohydrates, the monosaccharides, which may be monomers or part of a larger polymer. Monosaccharides are subunits that cannot be further hydrolysed in to simpler units. Depending on the number of carbon atom they are further classified into trioses, tetroses, pentoses, hexoses etc., which is further classified in to aldoses and ketoses depending on the type of functional group present in them.
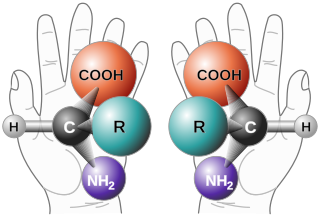
Chirality is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek χείρ (kheir), "hand", a familiar chiral object.
In chemical nomenclature, a descriptor is a notational prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some of the listed descriptors should not be used in publications, as they no longer accurately correspond with the recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.













