
A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus with the empirical formula Cm(H2O)n, which does not mean the H has covalent bonds with O. However, not all carbohydrates conform to this precise stoichiometric definition, nor are all chemicals that do conform to this definition automatically classified as carbohydrates.

Glucose is a sugar with the molecular formula C6H12O6. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living organisms to make adenosine triphosphate (ATP), which is used by the cell as energy.
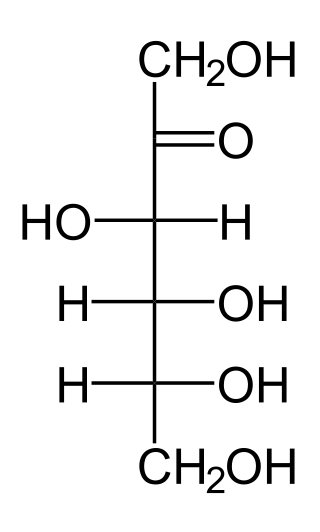
In chemistry, a hexose is a monosaccharide with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.

Mannose is a sugar with the formula HOCH2(CHOH)4CHO. It is one of the monomers of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule in order to form a glycoconjugate. In biology, glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation may refer to a non-enzymatic reaction.
In biochemistry, isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:

Galactokinase is an enzyme (phosphotransferase) that facilitates the phosphorylation of α-D-galactose to galactose 1-phosphate at the expense of one molecule of ATP. Galactokinase catalyzes the second step of the Leloir pathway, a metabolic pathway found in most organisms for the catabolism of α-D-galactose to glucose 1-phosphate. First isolated from mammalian liver, galactokinase has been studied extensively in yeast, archaea, plants, and humans.
In organic chemistry, a tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone group in position 2 (ketotetroses).
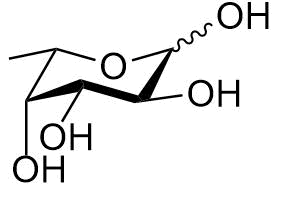
Fucose is a hexose deoxy sugar with the chemical formula C6H12O5. It is found on N-linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan. The α(1→3) linked core of fucoidan is a suspected carbohydrate antigen for IgE-mediated allergy.
Rhamnose is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L-arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as Pseudomonas aeruginosa and Helicobacter pylori.

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.
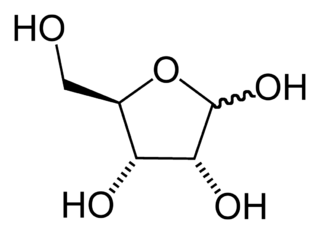
Deoxy sugars are sugars that have had a hydroxyl group replaced with a hydrogen atom.

Colitose is a mannose-derived 3,6-dideoxysugar produced by certain bacteria. It is a constituent of the lipopolysaccharide. It is the enantiomer of abequose.

Ribose 5-phosphate (R5P) is both a product and an intermediate of the pentose phosphate pathway. The last step of the oxidative reactions in the pentose phosphate pathway is the production of ribulose 5-phosphate. Depending on the body's state, ribulose 5-phosphate can reversibly isomerize to ribose 5-phosphate. Ribulose 5-phosphate can alternatively undergo a series of isomerizations as well as transaldolations and transketolations that result in the production of other pentose phosphates as well as fructose 6-phosphate and glyceraldehyde 3-phosphate.
In enzymology, an arabinose isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a dTDP-4-dehydrorhamnose 3,5-epimerase is an enzyme that catalyzes the chemical reaction
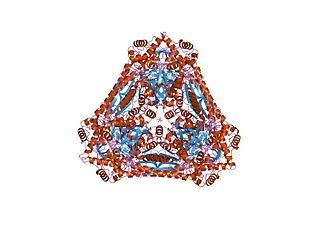
In enzymology, a L-fucose isomerase is an enzyme that catalyzes the chemical reaction
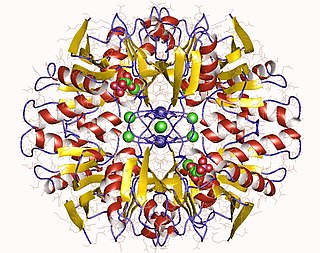
Ribose-5-phosphate isomerase (Rpi) encoded by the RPIA gene is an enzyme that catalyzes the conversion between ribose-5-phosphate (R5P) and ribulose-5-phosphate (Ru5P). It is a member of a larger class of isomerases which catalyze the interconversion of chemical isomers. It plays a vital role in biochemical metabolism in both the pentose phosphate pathway and the Calvin cycle. The systematic name of this enzyme class is D-ribose-5-phosphate aldose-ketose-isomerase.
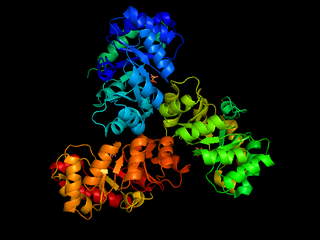
The enzyme 2-dehydro-3-deoxy-phosphogluconate aldolase, commonly known as KDPG aldolase, catalyzes the chemical reaction
The enzyme L-fuculose-phosphate aldolase (EC 4.1.2.17) catalyzes the chemical reaction















