
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.

Magnesium is a chemical element; it has symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium.

Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope, which is 103Rh. Naturally occurring rhodium is usually found as a free metal or as an alloy with similar metals and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals. Rhodium is a group 9 element.

Thallium is a chemical element; it has symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the international exhibition, which opened on 1 May that year.

Zirconium is a chemical element; it has symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, ductile, malleable and corrosion-resistant. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin.

The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.
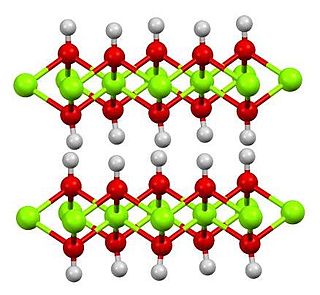
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10−12). Magnesium hydroxide is a common component of antacids, such as milk of magnesia.

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold. It is famous for its extreme oxidation properties. The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.
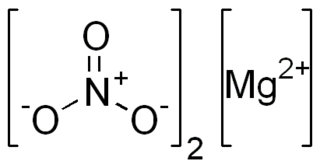
Magnesium nitrate refers to inorganic compounds with the formula Mg(NO3)2(H2O)x, where x = 6, 2, and 0. All are white solids. The anhydrous material is hygroscopic, quickly forming the hexahydrate upon standing in air. All of the salts are very soluble in both water and ethanol.
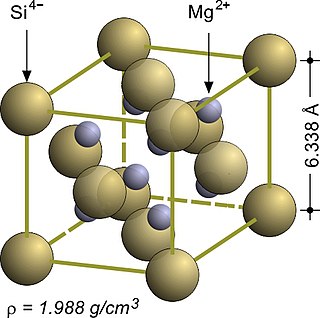
Magnesium silicide, Mg2Si, is an inorganic compound consisting of magnesium and silicon. As-grown Mg2Si usually forms black crystals; they are semiconductors with n-type conductivity and have potential applications in thermoelectric generators.

Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is an electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of most metals. As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory material. It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide, owing to its sweet taste.
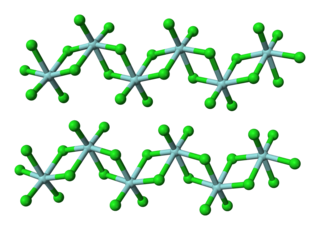
Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.

Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula Ta
2O
5. It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. Ta
2O
5 is an inert material with a high refractive index and low absorption, which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant.
Hafnium silicate is the hafnium(IV) salt of silicic acid with the chemical formula of HfSiO4.
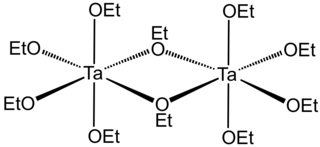
Tantalum(V) ethoxide is a metalorganic compound with formula Ta2(OC2H5)10, often abbreviated as Ta2(OEt)10. It is a colorless solid that dissolves in some organic solvents but hydrolyzes readily. It is used to prepare films of tantalum(V) oxide.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.




















