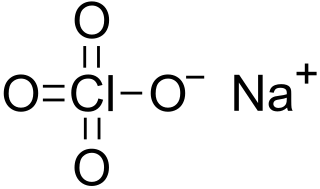Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
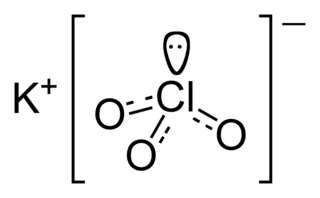
Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant.
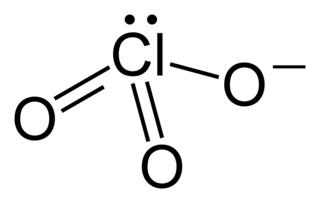
Chlorate is the common name of the ClO−
3 anion, whose chlorine atom is in the +5 oxidation state. The term can also refer to chemical compounds containing this anion, with chlorates being the salts of chloric acid. Other oxyanions of chlorine can be named "chlorate" followed by a Roman numeral in parentheses denoting the oxidation state of chlorine: e.g., the ClO−
4 ion commonly called perchlorate can also be called chlorate(VII).
Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.
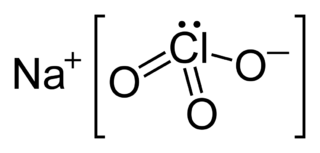
Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper.
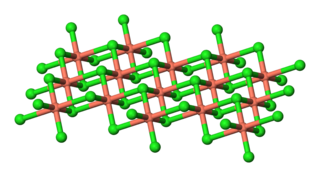
Copper(II) chloride, also known as cupric chloride, is an inorganic compound with the chemical formula CuCl2. The monoclinic yellowish-brown anhydrous form slowly absorbs moisture to form the orthorhombic blue-green dihydrate CuCl2·2H2O, with two water molecules of hydration. It is industrially produced for use as a co-catalyst in the Wacker process.
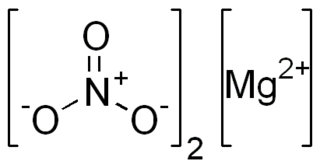
Magnesium nitrate refers to inorganic compounds with the formula Mg(NO3)2(H2O)x, where x = 6, 2, and 0. All are white solids. The anhydrous material is hygroscopic, quickly forming the hexahydrate upon standing in air. All of the salts are very soluble in both water and ethanol.
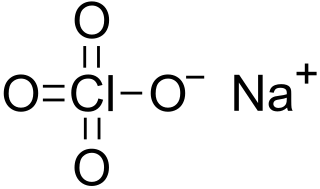
Sodium perchlorate is an inorganic compound with the chemical formula NaClO4. It consists of sodium cations Na+ and perchlorate anions ClO−4. It is a white crystalline, hygroscopic solid that is highly soluble in water and ethanol. It is usually encountered as sodium perchlorate monohydrate NaClO4·H2O. The compound is noteworthy as the most water-soluble of the common perchlorate salts.

Vanadium(III) chloride describes the inorganic compound with the formula VCl3 and its hydrates. It forms a purple anhydrous form and a green hexahydrate [VCl2(H2O)4]Cl·2H2O. These hygroscopic salts are common precursors to other vanadium(III) complexes and is used as a mild reducing agent.
Magnesium compounds are compounds formed by the element magnesium (Mg). These compounds are important to industry and biology, including magnesium carbonate, magnesium chloride, magnesium citrate, magnesium hydroxide, magnesium oxide, magnesium sulfate, and magnesium sulfate heptahydrate.
Calcium chlorate is the calcium salt of chloric acid, with the chemical formula Ca(ClO3)2. Like other chlorates, it is a strong oxidizer.
Magnesium hydroxychloride is the traditional term for several chemical compounds of magnesium, chlorine, oxygen, and hydrogen whose general formula xMgO·yMgCl2·zH2O, for various values of x, y, and z; or, equivalently, Mgx+y(OH)2xCl2y(H2O)z−x. The simple chemical formula that is often used is Mg(OH)Cl, which appears in high school subject, for example.Other names for this class are magnesium chloride hydroxide, magnesium oxychloride, and basic magnesium chloride. Some of these compounds are major components of Sorel cement.
Copper(II) chlorate is a chemical compound of the transition metal copper and the chlorate anion with basic formula Cu(ClO3)2. Copper chlorate is an oxidiser. It commonly forms the tetrahydrate, Cu(ClO3)2·4H2O.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.
Manganese(II) chlorate is an unstable chemical compound with the formula Mn(ClO3)2. It is unstable even in dilute solution. As a hexahydrate, it is solid below −18°C. Above this it melts, to form an extremely explosive pink liquid.
Silver chlorite is a chemical compound with the formula AgClO2. This slightly yellow solid is shock sensitive and has an orthorhombic crystal structure.
Magnesium permanganate is an inorganic compound with the chemical formula Mg(MnO4)2. It can be used as an oxidant.

Cobalt(II) perchlorate is an inorganic chemical compound with the formula Co(ClO4)2·nH2O (n = 0,6). The pink anhydrous and red hexahydrate forms are both hygroscopic solids.