A monopropellant rocket is a rocket that uses a single chemical as its propellant. Monopropellant rockets are commonly used as small attitude and trajectory control rockets in satellites, rocket upper stages, manned spacecraft, and spaceplanes.

A hypergolic propellant is a rocket propellant combination used in a rocket engine, whose components spontaneously ignite when they come into contact with each other.

Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.
Unsymmetrical dimethylhydrazine (abbreviated as UDMH; also known as 1,1-dimethylhydrazine, heptyl or Geptil) is a chemical compound with the formula H2NN(CH3)2 that is primarily used as a rocket propellant. At room temperature, UDMH is a colorless liquid, with a sharp, fishy, ammonia-like smell typical of organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It is miscible with water, ethanol, and kerosene. At concentrations between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock.
Monopropellants are propellants consisting of chemicals that release energy through exothermic chemical decomposition. The molecular bond energy of the monopropellant is released usually through use of a catalyst. This can be contrasted with bipropellants that release energy through the chemical reaction between an oxidizer and a fuel. While stable under defined storage conditions, monopropellants decompose very rapidly under certain other conditions to produce a large volume of its own energetic (hot) gases for the performance of mechanical work. Although solid deflagrants such as nitrocellulose, the most commonly used propellant in firearms, could be thought of as monopropellants, the term is usually reserved for liquids in engineering literature.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.
A propellant is a mass that is expelled or expanded in such a way as to create a thrust or another motive force in accordance with Newton's third law of motion, and "propel" a vehicle, projectile, or fluid payload. In vehicles, the engine that expels the propellant is called a reaction engine. Although technically a propellant is the reaction mass used to create thrust, the term "propellant" is often used to describe a substance which contains both the reaction mass and the fuel that holds the energy used to accelerate the reaction mass. For example, the term "propellant" is often used in chemical rocket design to describe a combined fuel/propellant, although the propellants should not be confused with the fuel that is used by an engine to produce the energy that expels the propellant. Even though the byproducts of substances used as fuel are also often used as a reaction mass to create the thrust, such as with a chemical rocket engine, propellant and fuel are two distinct concepts.
Monomethylhydrazine (MMH) is a highly toxic, volatile hydrazine derivative with the chemical formula CH6N2. It is used as a rocket propellant in bipropellant rocket engines because it is hypergolic with various oxidizers such as nitrogen tetroxide and nitric acid. As a propellant, it is described in specification MIL-PRF-27404.
Acrylonitrile is an organic compound with the formula CH2CHCN and the structure H2C=CH−C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group linked to a nitrile. It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.
The highest specific impulse chemical rockets use liquid propellants. They can consist of a single chemical or a mix of two chemicals, called bipropellants. Bipropellants can further be divided into two categories; hypergolic propellants, which ignite when the fuel and oxidizer make contact, and non-hypergolic propellants which require an ignition source.
The peroxide process is a method for the industrial production of hydrazine.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is a colorless, poisonous, corrosive, and extremely reactive gas that condenses to a pale-greenish yellow liquid, the form in which it is most often sold. It is famous for its extreme oxidation properties. The compound is primarily of interest in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, historically as a component in rocket fuels, and various other industrial operations owing to its corrosive nature.
A gas generator is a device for generating gas. A gas generator may create gas by a chemical reaction or from a solid or liquid source, when storing a pressurized gas is undesirable or impractical.

Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
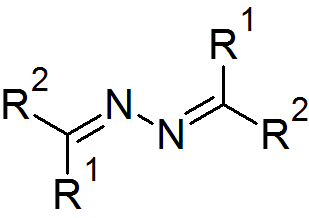
Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, nonflammable, reactive inorganic gas. It is a fluorinated analog of hydrazine.
Nitrous oxide fuel blend propellants are a class of liquid rocket propellants that were intended in the early 2010s to be able to replace hydrazine as the standard storable rocket propellent in some applications.

Acetone azine is the simplest ketazine. It is an intermediate in some hydrazine manufacturing processes.




















