
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.
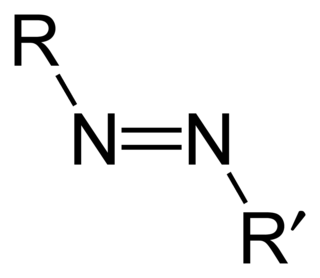
Azo compounds are organic compounds bearing the functional group diazenyl.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. The reaction was reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912.
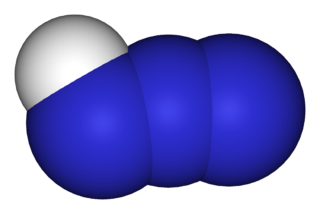
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
In chemistry, a trimer is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization.

Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.
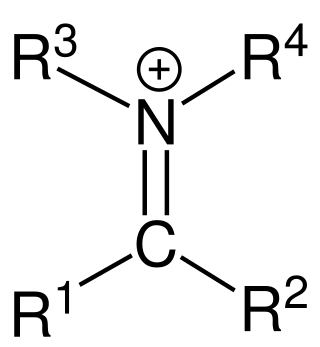
In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.
Hydrazides in organic chemistry are a class of organic compounds with the formula R−NR1−NR2R3 where R is acyl, sulfonyl, phosphoryl, phosphonyl and similar groups, R1, R2, R3 and R' are any groups. Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive influence of the acyl, sulfonyl, or phosphoryl substituent.

Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula [H3O]2[PtCl6](H2O)x (0 ≤ x ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Although often written in shorthand as H2PtCl6, it is the hydronium (H3O+) salt of the hexachloroplatinate anion (PtCl2−
6). Hexachloroplatinic acid is highly hygroscopic.
p-Toluenesulfonyl hydrazide is the organic compound with the formula CH3C6H4SO2NHNH2. It is a white solid that is soluble in many organic solvents but not water or alkanes. It is a reagent in organic synthesis.
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular H2. It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular H2 used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to CO2, acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen, chalcogen, or halogen. The oldest-known onium ion, and the namesake for the class, is ammonium, NH+4, the protonated derivative of ammonia, NH3.
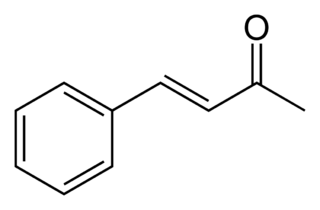
Benzylideneacetone is the organic compound described by the formula C6H5CH=CHC(O)CH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed. Its original preparation demonstrated the scope of condensation reactions to construct new, complex organic compounds. Benzylideneacetone is used as a flavouring ingredient in food and perfumes.
The Milas hydroxylation is an organic reaction converting an alkene to a vicinal diol, and was developed by Nicholas A. Milas in the 1930s. The cis-diol is formed by reaction of alkenes with hydrogen peroxide and either ultraviolet light or a catalytic osmium tetroxide, vanadium pentoxide, or chromium trioxide.
Reductions with diimide are a chemical reactions that convert unsaturated organic compounds to reduced alkane products. In the process, diimide is oxidized to dinitrogen.
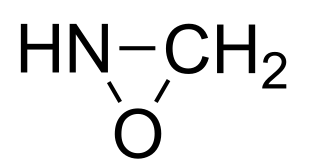
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to nitrones and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
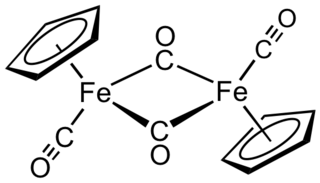
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
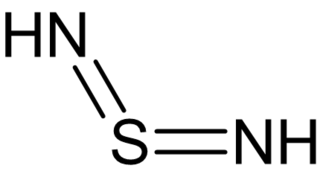
Sulfur diimides are chemical compounds of the formula S(NR)2. Structurally, they are the diimine of sulfur dioxide. The parent member, S(NH)2, is of only theoretical interest. Other derivatives where R is an organic group are stable and useful reagents.
In organic chemistry, the Myers allene synthesis is a chemical reaction that converts a propargyl alcohol into an allene by way of an arenesulfonylhydrazine as a key intermediate. This name reaction is one of two discovered by Andrew Myers that are named after him; both this reaction and the Myers deoxygenation reaction involve the same type of intermediate.
Cobalt compounds are chemical compounds formed by cobalt with other elements.














