
Bischofite is a hydrous magnesium chloride mineral with formula MgCl2·6H2O. It belongs to halides and is a sea salt concentrate. It contains many macro- and micro-elements vital for human health, in much higher concentrations than can be found in sea or ocean salt. The main bischofite compound is magnesium chloride (up to 350 g/L), moreover, it contains about 70 other elements as impurities, including potassium, sodium, bromine, boron, calcium, silicon, molybdenum, silver, zinc, iron and copper.

The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts. Sulfide minerals are inorganic compounds.

The Borate Minerals are minerals which contain a borate anion group. The borate (BO3) units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures which include hydroxide or halogen anions. The [B(O,OH)4]− anion exists as well.

Phosphate minerals contain the tetrahedrally coordinated phosphate (PO43−) anion, sometimes with arsenate (AsO43−) and vanadate (VO43−) substitutions, along with chloride (Cl−), fluoride (F−), and hydroxide (OH−) anions, that also fit into the crystal structure.

Carbonate minerals are those minerals containing the carbonate ion, CO2−
3.
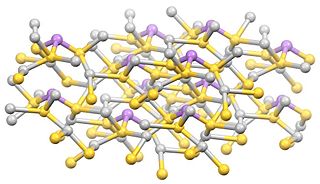
Sulfosalt minerals are sulfide minerals with the general formula AmBnXp, where
Arsenate minerals usually refer to the naturally occurring orthoarsenates, possessing the (AsO4)3− anion group and, more rarely, other arsenates with anions like AsO3(OH)2− (also written HAsO42−) (example: pharmacolite Ca(AsO3OH).2H2O) or (very rarely) [AsO2(OH)2]− (example: andyrobertsite). Arsenite minerals are much less common. Both the Dana and the Strunz mineral classifications place the arsenates in with the phosphate minerals.

The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. Minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately.

Nickel–Strunz classification is a scheme for categorizing minerals based upon their chemical composition, introduced by German mineralogist Karl Hugo Strunz in his Mineralogische Tabellen (1941). The 4th and the 5th edition was also edited by Christel Tennyson (1966). It was followed by A.S. Povarennykh with a modified classification.
Arsenite minerals are very rare oxygen-bearing arsenic minerals. Classical world localities where such minerals occur include the complex skarn manganese deposit at Långban (Sweden) and the polymetallic Tsumeb deposit (Namibia). The most often reported arsenite anion in minerals is the AsO33− anion, present for example in reinerite Zn3(AsO3)2. Unique diarsenite anions occur i. e. in leiteite Zn[As2O4] and paulmooreite Pb[As2O5]. More complex arsenites include schneiderhöhnite Fe2+Fe3+3[As5O13] and ludlockite PbFe3+4As10O22.
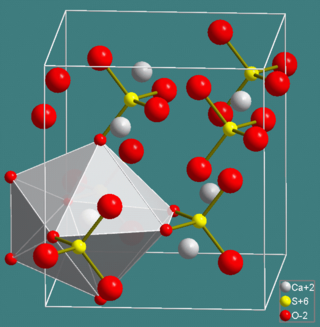
The sulfate minerals are a class of minerals that include the sulfate ion within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.

Native element minerals are those elements that occur in nature in uncombined form with a distinct mineral structure. The elemental class includes metals, intermetallic compounds, alloys, metalloids, and nonmetals. The Nickel–Strunz classification system also includes the naturally occurring phosphides, silicides, nitrides, carbides, and arsenides.
Some organic compounds are valid minerals, recognized by the CNMNC (IMA).

Ferronickel platinum is a very rarely occurring minerals from the mineral class of elements (including natural alloys, intermetallic compounds, carbides, nitrides, phosphides and silicides) with the chemical composition Pt2FeNi and thus is chemically seen as a natural alloy, more precisely an intermetallic compound of platinum, nickel and iron in a ratio of 2:1:1.













