
The hypothalamus is a small part of the vertebrate brain that contains a number of nuclei with a variety of functions. One of the most important functions is to link the nervous system to the endocrine system via the pituitary gland. The hypothalamus is located below the thalamus and is part of the limbic system. It forms the basal part of the diencephalon. All vertebrate brains contain a hypothalamus. In humans, it is about the size of an almond.
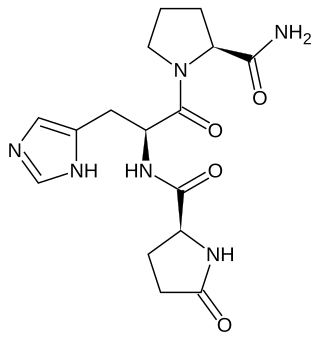
Thyrotropin-releasing hormone (TRH) is a hypophysiotropic hormone produced by neurons in the hypothalamus that stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary.

The hypothalamic–pituitary–adrenal axis is a complex set of direct influences and feedback interactions among three components: the hypothalamus, the pituitary gland, and the adrenal glands. These organs and their interactions constitute the HPS axis.
Corticotropin-releasing factor family, CRF family is a family of related neuropeptides in vertebrates. This family includes corticotropin-releasing hormone, urotensin-I, urocortin, and sauvagine. The family can be grouped into 2 separate paralogous lineages, with urotensin-I, urocortin and sauvagine in one group and CRH forming the other group. Urocortin and sauvagine appear to represent orthologues of fish urotensin-I in mammals and amphibians, respectively. The peptides have a variety of physiological effects on stress and anxiety, vasoregulation, thermoregulation, growth and metabolism, metamorphosis and reproduction in various species, and are all released as prohormones.
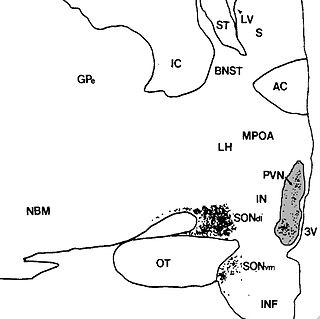
The paraventricular nucleus is a nucleus in the hypothalamus. Anatomically, it is adjacent to the third ventricle and many of its neurons project to the posterior pituitary. These projecting neurons secrete oxytocin and a smaller amount of vasopressin, otherwise the nucleus also secretes corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH). CRH and TRH are secreted into the hypophyseal portal system and act on different targets neurons in the anterior pituitary. Dysfunctions of PVN can cause hypersomnia in mice, and dysfunction of the paraventricular nucleus can lead to drowsiness for up to 20 hours per day in humans. PVN is thought to mediate many diverse functions through different hormones, including osmoregulation, appetite,wakefulness, and the response of the body to stress.
Corticotropic cells, are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.

Ghrelin is a hormone primarily produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are highest before meals when hungry, returning to lower levels after mealtimes. Ghrelin may help prepare for food intake by increasing gastric motility and stimulating the secretion of gastric acid.

Neuropeptide Y (NPY) is a 36 amino-acid neuropeptide that is involved in various physiological and homeostatic processes in both the central and peripheral nervous systems. It is secreted alongside other neurotransmitters such as GABA and glutamate.
Neuroendocrinology is the branch of biology which studies the interaction between the nervous system and the endocrine system; i.e. how the brain regulates the hormonal activity in the body. The nervous and endocrine systems often act together in a process called neuroendocrine integration, to regulate the physiological processes of the human body. Neuroendocrinology arose from the recognition that the brain, especially the hypothalamus, controls secretion of pituitary gland hormones, and has subsequently expanded to investigate numerous interconnections of the endocrine and nervous systems.
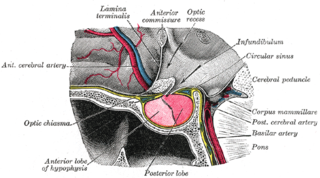
The hypophyseal portal system is a system of blood vessels in the microcirculation at the base of the brain, connecting the hypothalamus with the anterior pituitary. Its main function is to quickly transport and exchange hormones between the hypothalamus arcuate nucleus and anterior pituitary gland. The capillaries in the portal system are fenestrated which allows a rapid exchange between the hypothalamus and the pituitary. The main hormones transported by the system include gonadotropin-releasing hormone, corticotropin-releasing hormone, growth hormone–releasing hormone, and thyrotropin-releasing hormone.

Urocortin is a protein that in humans is encoded by the UCN gene. Urocortin belongs to the corticotropin-releasing factor (CRF) family of proteins which includes CRF, urotensin I, sauvagine, urocortin II and urocortin III. Urocortin is involved in the mammalian stress response, and regulates aspects of appetite and stress response.
Urocortin III, a 38–41 amino acid peptide, is a member of the CRF, also known as CRH family of peptides, with a long evolutionary lineage.
Neuromedin U is a neuropeptide found in the brain of humans and other mammals, which has a number of diverse functions including contraction of smooth muscle, regulation of blood pressure, pain perception, appetite, bone growth, and hormone release. It was first isolated from the spinal cord in 1985, and named after its ability to cause smooth muscle contraction in the uterus.

Corticotropin-releasing hormone receptor 1 (CRHR1) is a protein, also known as CRF1, with the latter (CRF1) now being the IUPHAR-recommended name. In humans, CRF1 is encoded by the CRHR1 gene at region 17q21.31, beside micrototubule-associated protein tau MAPT.
Corticotropin-releasing hormone receptor 2 (CRHR2) is a protein, also known by the IUPHAR-recommended name CRF2, that is encoded by the CRHR2 gene and occurs on the surfaces of some mammalian cells. CRF2 receptors are type 2 G protein-coupled receptors for corticotropin-releasing hormone (CRH) that are resident in the plasma membranes of hormone-sensitive cells. CRH, a peptide of 41 amino acids synthesized in the hypothalamus, is the principal neuroregulator of the hypothalamic-pituitary-adrenal axis, signaling via guanine nucleotide-binding proteins (G proteins) and downstream effectors such as adenylate cyclase. The CRF2 receptor is a multi-pass membrane protein with a transmembrane domain composed of seven helices arranged in a V-shape. CRF2 receptors are activated by two structurally similar peptides, urocortin II, and urocortin III, as well as CRH.

Corticotropin-releasing factor-binding protein is a protein that in humans is encoded by the CRHBP gene. It belongs to corticotropin-releasing hormone binding protein family.
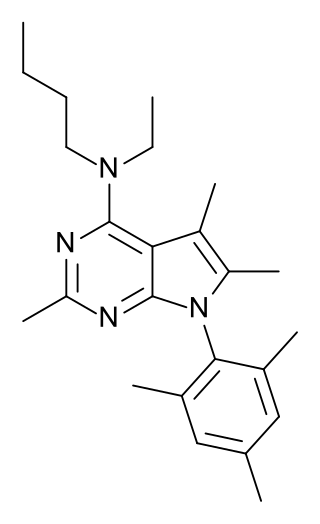
Antalarmin (CP-156,181) is a drug that acts as a CRH1 antagonist.
A Corticotropin-releasing hormone antagonist is a specific type of receptor antagonist that blocks the receptor sites for corticotropin-releasing hormone, also known as corticotropin-releasing factor (CRF), which synchronizes the behavioral, endocrine, autonomic, and immune responses to stress by controlling the hypothalamic-pituitary-adrenal axis. CRH antagonists thereby block the consequent secretions of ACTH and cortisol due to stress, among other effects.
Parvocellular neurosecretory cells are small neurons that produce hypothalamic releasing and inhibiting hormones. The cell bodies of these neurons are located in various nuclei of the hypothalamus or in closely related areas of the basal brain, mainly in the medial zone of the hypothalamus. All or most of the axons of the parvocellular neurosecretory cells project to the median eminence, at the base of the brain, where their nerve terminals release the hypothalamic hormones. These hormones are then immediately absorbed into the blood vessels of the hypothalamo-pituitary portal system, which carry them to the anterior pituitary gland, where they regulate the secretion of hormones into the systemic circulation.
Gonadotropin-inhibitory hormone (GnIH) is a RFamide-related peptide coded by the NPVF gene in mammals.




















