
Acinetobacter is a genus of Gram-negative bacteria belonging to the wider class of Gammaproteobacteria. Acinetobacter species are oxidase-negative, exhibit twitching motility, and occur in pairs under magnification.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
Multiple drug resistance (MDR), multidrug resistance or multiresistance is antimicrobial resistance shown by a species of microorganism to at least one antimicrobial drug in three or more antimicrobial categories. Antimicrobial categories are classifications of antimicrobial agents based on their mode of action and specific to target organisms. The MDR types most threatening to public health are MDR bacteria that resist multiple antibiotics; other types include MDR viruses, parasites.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. P. aeruginosa is able to selectively inhibit various antibiotics from penetrating its outer membrane - and has high resistance to several antibiotics. According to the World Health Organization P. aeruginosa poses one of the greatest threats to humans in terms of antibiotic resistance.
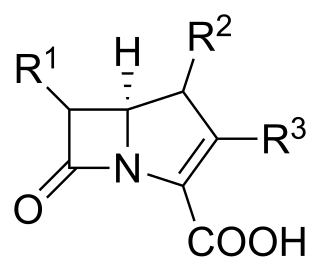
Carbapenems are a class of very effective antibiotic agents most commonly used for treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta-lactam antibiotics drug class, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.

Imipenem is a synthetic β-lactam antibiotic belonging to the carbapenems chemical class. developed by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s. Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, thus playing a key role in the treatment of infections not readily treated with other antibiotics. It is usually administered through intravenous injection.

Amikacin is an antibiotic medication used for a number of bacterial infections. This includes joint infections, intra-abdominal infections, meningitis, pneumonia, sepsis, and urinary tract infections. It is also used for the treatment of multidrug-resistant tuberculosis. It is used by injection into a vein using an IV or into a muscle.

Acinetobacter baumannii is a typically short, almost round, rod-shaped (coccobacillus) Gram-negative bacterium. It is named after the bacteriologist Paul Baumann. It can be an opportunistic pathogen in humans, affecting people with compromised immune systems, and is becoming increasingly important as a hospital-derived (nosocomial) infection. While other species of the genus Acinetobacter are often found in soil samples, it is almost exclusively isolated from hospital environments. Although occasionally it has been found in environmental soil and water samples, its natural habitat is still not known.
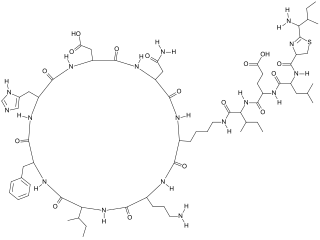
Polypeptide antibiotics are a chemically diverse class of anti-infective and antitumor antibiotics containing non-protein polypeptide chains. Examples of this class include actinomycin, bacitracin, colistin, and polymyxin B. Actinomycin-D has found use in cancer chemotherapy. Most other polypeptide antibiotics are too toxic for systemic administration, but can safely be administered topically to the skin as an antiseptic for shallow cuts and abrasions.
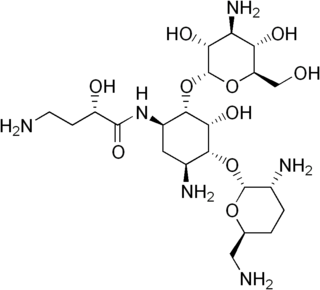
Arbekacin (INN) is a semisynthetic aminoglycoside antibiotic which was derived from kanamycin. It is primarily used for the treatment of infections caused by multi-resistant bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Arbekacin was originally synthesized from dibekacin in 1973 by Hamao Umezawa and collaborators. It has been registered and marketed in Japan since 1990 under the trade name Habekacin. Arbekacin is no longer covered by patent and generic versions of the drug are also available under such trade names as Decontasin and Blubatosine.
Multidrug resistant Gram-negative bacteria are a type of Gram-negative bacteria with resistance to multiple antibiotics. They can cause bacteria infections that pose a serious and rapidly emerging threat for hospitalized patients and especially patients in intensive care units. Infections caused by MDR strains are correlated with increased morbidity, mortality, and prolonged hospitalization. Thus, not only do these bacteria pose a threat to global public health, but also create a significant burden to healthcare systems.

Eravacycline is a synthetic halogenated tetracycline class antibiotic by Tetraphase Pharmaceuticals. It is closely related to tigecycline. It has a broad spectrum of activity including many multi-drug resistant strains of bacteria. Phase III studies in complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI) were recently completed with mixed results. Eravacycline was granted fast track designation by the FDA and is currently available in USA.
The mobilized colistin resistance (mcr) gene confers plasmid-mediated resistance to colistin, one of a number of last-resort antibiotics for treating Gram-negative infections. mcr-1, the original variant, is capable of horizontal transfer between different strains of a bacterial species. After discovery in November 2015 in E. coli from a pig in China it has been found in Escherichia coli, Salmonella enterica, Klebsiella pneumoniae, Enterobacter aerogenes, and Enterobacter cloacae. As of 2017, it has been detected in more than 30 countries on 5 continents in less than a year.
ESKAPE is an acronym comprising the scientific names of six highly virulent and antibiotic resistant bacterial pathogens including: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. The acronym is sometimes extended to ESKAPEE to include Escherichia coli. This group of Gram-positive and Gram-negative bacteria can evade or 'escape' commonly used antibiotics due to their increasing multi-drug resistance (MDR). As a result, throughout the world, they are the major cause of life-threatening nosocomial or hospital-acquired infections in immunocompromised and critically ill patients who are most at risk. P. aeruginosa and S. aureus are some of the most ubiquitous pathogens in biofilms found in healthcare. P. aeruginosa is a Gram-negative, rod-shaped bacterium, commonly found in the gut flora, soil, and water that can be spread directly or indirectly to patients in healthcare settings. The pathogen can also be spread in other locations through contamination, including surfaces, equipment, and hands. The opportunistic pathogen can cause hospitalized patients to have infections in the lungs, blood, urinary tract, and in other body regions after surgery. S. aureus is a Gram-positive, cocci-shaped bacterium, residing in the environment and on the skin and nose of many healthy individuals. The bacterium can cause skin and bone infections, pneumonia, and other types of potentially serious infections if it enters the body. S. aureus has also gained resistance to many antibiotic treatments, making healing difficult. Because of natural and unnatural selective pressures and factors, antibiotic resistance in bacteria usually emerges through genetic mutation or acquires antibiotic-resistant genes (ARGs) through horizontal gene transfer - a genetic exchange process by which antibiotic resistance can spread.

Murepavadin also known as POL7080 is a Pseudomonas specific peptidomimetic antibiotic. It is a synthetic cyclic beta hairpin peptidomimetic based on the cationic antimicrobial peptide protegrin I (PG-1) and the first example of an outer membrane protein-targeting antibiotic class with a novel, nonlytic mechanism of action, highly active and selective against the protein transporter LptD of Pseudomonas aeruginosa. In preclinical studies the compound was highly active on a broad panel of clinical isolates including multi-drug resistant Pseudomonas bacteria with outstanding in vivo efficacy in sepsis, lung, and thigh infection models. Intravenous murepavadin is in development for the treatment of bacterial hospital-acquired pneumonia and bacterial ventilator-associated pneumonia due to Pseudomonas aeruginosa.

Cefiderocol, sold under the brand name Fetroja among others, is an antibiotic used to treat complicated urinary tract infections when no other options are available. It is indicated for the treatment of multi-drug-resistant Gram-negative bacteria including Pseudomonas aeruginosa. It is given by injection into a vein.

The Center for Innovative Phage Applications and Therapeutics (IPATH) is the first phage therapy center in North America, founded in the UC San Diego School of Medicine in June 2018, with seed funding from UC San Diego Chancellor Pradeep Khosla. The center was founded by Steffanie A. Strathdee and Robert "Chip" Schooley, both professors at UC San Diego School of Medicine. The center currently treats patients with life-threatening multi-drug resistant infections with phage therapy, on a case-by-case basis, through the Food and Drug Administration's (FDA's) compassionate use program. IPATH aims to initiate phase I/II phage therapy clinical trials, focusing on patients with cystic fibrosis and infections related to implantable hardware, such as pacemakers and prosthetic joints. The first planned clinical trial is set to look at otherwise healthy cystic fibrosis patients that are shedding Pseudomonas aeruginosa.

Sulbactam/durlobactam, sold under the brand name Xacduro, is a co-packaged medication used for the treatment of bacterial pneumonia caused by Acinetobacter baumannii-calcoaceticus complex. It contains sulbactam, a beta-lactam antibacterial and beta-lactamase inhibitor; and durlobactam, a beta-lactamase inhibitor.

SPR741 is an experimental antibiotic related to Polymyxin B. It shows activity against a number of bacterial pathogens, especially Acinetobacter baumannii and Klebsiella pneumoniae, acting as an antibiotic adjuvant which disrupts the outer membrane of Gram-negative bacteria and allows other antibiotics to more effectively penetrate into the cell.


















