
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Cytochrome P450 1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the human body. In humans, the CYP1A2 enzyme is encoded by the CYP1A2 gene.
The epoxyeicosatrienoic acids or EETs are signaling molecules formed within various types of cells by the metabolism of arachidonic acid by a specific subset of Cytochrome P450 enzymes termed cytochrome P450 epoxygenases. These nonclassic eicosanoids are generally short-lived, being rapidly converted from epoxides to less active or inactive dihydroxy-eicosatrienoic acids (diHETrEs) by a widely distributed cellular enzyme, Soluble epoxide hydrolase (sEH), also termed Epoxide hydrolase 2. The EETs consequently function as transiently acting, short-range hormones; that is, they work locally to regulate the function of the cells that produce them or of nearby cells. The EETs have been most studied in animal models where they show the ability to lower blood pressure possibly by a) stimulating arterial vasorelaxation and b) inhibiting the kidney's retention of salts and water to decrease intravascular blood volume. In these models, EETs prevent arterial occlusive diseases such as heart attacks and brain strokes not only by their anti-hypertension action but possibly also by their anti-inflammatory effects on blood vessels, their inhibition of platelet activation and thereby blood clotting, and/or their promotion of pro-fibrinolytic removal of blood clots. With respect to their effects on the heart, the EETs are often termed cardio-protective. Beyond these cardiovascular actions that may prevent various cardiovascular diseases, studies have implicated the EETs in the pathological growth of certain types of cancer and in the physiological and possibly pathological perception of neuropathic pain. While studies to date imply that the EETs, EET-forming epoxygenases, and EET-inactivating sEH can be manipulated to control a wide range of human diseases, clinical studies have yet to prove this. Determination of the role of the EETS in human diseases is made particularly difficult because of the large number of EET-forming epoxygenases, large number of epoxygenase substrates other than arachidonic acid, and the large number of activities, some of which may be pathological or injurious, that the EETs possess.
Cytochrome P450 family 2 subfamily C member 9 is an enzyme protein. The enzyme is involved in metabolism, by oxidation, of both xenobiotics, including drugs, and endogenous compounds, including fatty acids. In humans, the protein is encoded by the CYP2C9 gene. The gene is highly polymorphic, which affects the efficiency of the metabolism by the enzyme.

Cytochrome P4502C8 (abbreviated CYP2C8), a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the body. Cytochrome P4502C8 also possesses epoxygenase activity, i.e. it metabolizes long-chain polyunsaturated fatty acids, e.g. arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and Linoleic acid to their biologically active epoxides.
Omega oxidation (ω-oxidation) is a process of fatty acid metabolism in some species of animals. It is an alternative pathway to beta oxidation that, instead of involving the β carbon, involves the oxidation of the ω carbon. The process is normally a minor catabolic pathway for medium-chain fatty acids, but becomes more important when β oxidation is defective.

Cytochrome P450, family 1, subfamily A, polypeptide 1 is a protein that in humans is encoded by the CYP1A1 gene. The protein is a member of the cytochrome P450 superfamily of enzymes.

Cytochrome P450 2C18 is a protein that in humans is encoded by the CYP2C18 gene.

Cytochrome P450 4A11 is a protein that in humans is codified by the CYP4A11 gene.

Cytochrome P450 2S1 is a protein that in humans is encoded by the CYP2S1 gene. The gene is located in chromosome 19q13.2 within a cluster including other CYP2 family members such as CYP2A6, CYP2A13, CYP2B6, and CYP2F1.

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Cytochrome P450 4F12 is a protein that in humans is encoded by the CYP4F12 gene.
Epoxygenases are a set of membrane-bound, heme-containing cytochrome P450 enzymes that metabolize polyunsaturated fatty acids to epoxide products that have a range of biological activities. The most thoroughly studied substrate of the CYP epoxylgenases is arachidonic acid. This polyunsaturated fatty acid is metabolized by cyclooxygenases to various prostaglandin, thromboxane, and prostacyclin metabolites in what has been termed the first pathway of eicosanoid production; it is also metabolized by various lipoxygenases to hydroxyeicosatetraenoic acids and leukotrienes in what has been termed the second pathway of eicosanoid production. The metabolism of arachidonic acid to epoxyeicosatrienoic acids by the CYP epoxygenases has been termed the third pathway of eicosanoid metabolism. Like the first two pathways of eicosanoid production, this third pathway acts as a signaling pathway wherein a set of enzymes metabolize arachidonic acid to a set of products that act as secondary signals to work in activating their parent or nearby cells and thereby orchestrate functional responses. However, none of these three pathways is limited to metabolizing arachidonic acid to eicosanoids. Rather, they also metabolize other polyunsaturated fatty acids to products that are structurally analogous to the eicosanoids but often have different bioactivity profiles. This is particularly true for the CYP epoxygenases which in general act on a broader range of polyunsaturated fatty acids to form a broader range of metabolites than the first and second pathways of eicosanoid production. Furthermore, the latter pathways form metabolites many of which act on cells by binding with and thereby activating specific and well-characterized receptor proteins; no such receptors have been fully characterized for the epoxide metabolites. Finally, there are relatively few metabolite-forming lipoxygenases and cyclooxygenases in the first and second pathways and these oxygenase enzymes share similarity between humans and other mammalian animal models. The third pathway consists of a large number of metabolite-forming CYP epoxygenases and the human epoxygenases have important differences from those of animal models. Partly because of these differences, it has been difficult to define clear roles for the epoxygenase-epoxide pathways in human physiology and pathology.

CYP4Z1 is a protein that in humans is encoded by the CYP4Z1 gene.

CYP4F11 is a protein that in humans is encoded by the CYP4F11 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This gene is part of a cluster of cytochrome P450 genes on chromosome 19. Another member of this family, CYP4F2, is approximately 16 kb away. Alternatively spliced transcript variants encoding the same protein have been found for this gene.
Soluble epoxide hydrolase (sEH) is a bifunctional enzyme that in humans is encoded by the EPHX2 gene. sEH is a member of the epoxide hydrolase family. This enzyme, found in both the cytosol and peroxisomes, binds to specific epoxides and converts them to the corresponding diols. A different region of this protein also has lipid-phosphate phosphatase activity. Mutations in the EPHX2 gene have been associated with familial hypercholesterolemia.

20-Hydroxyeicosatetraenoic acid, also known as 20-HETE or 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid, is an eicosanoid metabolite of arachidonic acid that has a wide range of effects on the vascular system including the regulation of vascular tone, blood flow to specific organs, sodium and fluid transport in the kidney, and vascular pathway remodeling. These vascular and kidney effects of 20-HETE have been shown to be responsible for regulating blood pressure and blood flow to specific organs in rodents; genetic and preclinical studies suggest that 20-HETE may similarly regulate blood pressure and contribute to the development of stroke and heart attacks. Additionally the loss of its production appears to be one cause of the human neurological disease, Hereditary spastic paraplegia. Preclinical studies also suggest that the overproduction of 20-HETE may contribute to the progression of certain human cancers, particularly those of the breast.

Coronaric acid (isoleukotoxin) is a mono-unsaturated, epoxide derivative of the di-saturated fatty acid, linoleic acid (i.e. 9 ,12 octadecadienoic acid. It is a mixture of the two optically active isomers of 12 9,10-epoxy-octadecenoic acid. This mixture is also termed 9,10-epoxy-12Z-octadecenoic acid or 9 -EpOME and when formed by or studied in mammalians, isoleukotoxin.
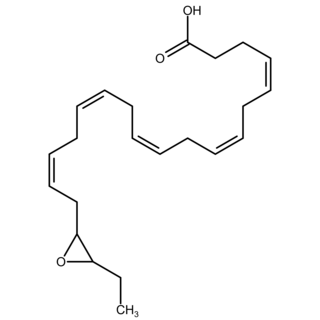
Epoxide docosapentaenoic acids are metabolites of the 22-carbon straight-chain omega-3 fatty acid, docosahexaenoic acid (DHA). Cell types that express certain cytochrome P450 (CYP) epoxygenases metabolize polyunsaturated fatty acid's (PUFAs) by converting one of their double bonds to an epoxide. In the best known of these metabolic pathways, cellular CYP epoxygenases metabolize the 20-carbon straight-chain omega-6 fatty acid, arachidonic acid, to epoxyeicosatrienoic acids (EETs); another CYP epoxygenase pathway metabolizes the 20-carbon omega-3 fatty acid, eicosapentaenoic acid (EPA), to epoxyeicosatetraenoic acids (EEQs). CYP epoxygenases similarly convert various other PUFAs to epoxides These epoxide metabolites have a variety of activities. However, essentially all of them are rapidly converted to their corresponding, but in general far less active, Vicinal (chemistry) dihydroxy fatty acids by ubiquitous cellular Soluble epoxide hydrolase. Consequently, these epoxides, including EDPs, operate as short-lived signaling agents that regulate the function of their parent or nearby cells. The particular feature of EDPs distinguishing them from EETs is that they derive from omega-3 fatty acids and are suggested to be responsible for some of the beneficial effects attributed to omega-3 fatty acids and omega-3-rich foods such as fish oil.
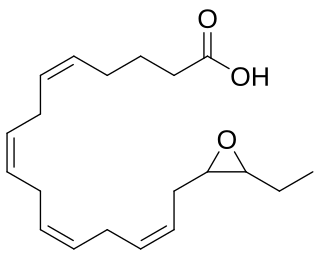
Epoxyeicosatetraenoic acids are a set of biologically active epoxides that various cell types make by metabolizing the omega 3 fatty acid, eicosapentaenoic acid (EPA), with certain cytochrome P450 epoxygenases. These epoxygenases can metabolize EPA to as many as 10 epoxides that differ in the site and/or stereoisomer of the epoxide formed; however, the formed EEQs, while differing in potency, often have similar bioactivities and are commonly considered together.















