The Enzyme Commission number is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze. As a system of enzyme nomenclature, every EC number is associated with a recommended name for the corresponding enzyme-catalyzed reaction.

Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Angiotensin also stimulates the release of aldosterone from the adrenal cortex to promote sodium retention by the kidneys.
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.

Angiotensin-converting enzyme, or ACE, is a central component of the renin–angiotensin system (RAS), which controls blood pressure by regulating the volume of fluids in the body. It converts the hormone angiotensin I to the active vasoconstrictor angiotensin II. Therefore, ACE indirectly increases blood pressure by causing blood vessels to constrict. ACE inhibitors are widely used as pharmaceutical drugs for treatment of cardiovascular diseases.

Epoxide hydrolases (EH's), also known as epoxide hydratases, are enzymes that metabolize compounds that contain an epoxide residue; they convert this residue to two hydroxyl residues through an epoxide hydrolysis reaction to form diol products. Several enzymes possess EH activity. Microsomal epoxide hydrolase, soluble epoxide hydrolase, and the more recently discovered but not as yet well defined functionally, epoxide hydrolase 3 (EH3) and epoxide hydrolase 4 (EH4) are structurally closely related isozymes. Other enzymes with epoxide hydrolase activity include leukotriene A4 hydrolase, Cholesterol-5,6-oxide hydrolase, MEST (gene) (Peg1/MEST), and Hepoxilin-epoxide hydrolase. The hydrolases are distinguished from each other by their substrate preferences and, directly related to this, their functions.
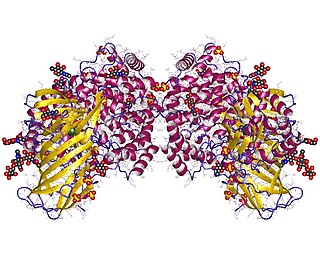
Membrane alanyl aminopeptidase also known as alanyl aminopeptidase (AAP) or aminopeptidase N (AP-N) is an enzyme that in humans is encoded by the ANPEP gene.
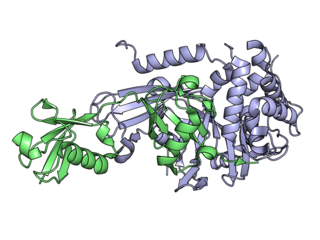
Deubiquitinating enzymes (DUBs), also known as deubiquitinating peptidases, deubiquitinating isopeptidases, deubiquitinases, ubiquitin proteases, ubiquitin hydrolases, ubiquitin isopeptidases, are a large group of proteases that cleave ubiquitin from proteins. Ubiquitin is attached to proteins in order to regulate the degradation of proteins via the proteasome and lysosome; coordinate the cellular localisation of proteins; activate and inactivate proteins; and modulate protein-protein interactions. DUBs can reverse these effects by cleaving the peptide or isopeptide bond between ubiquitin and its substrate protein. In humans there are nearly 100 DUB genes, which can be classified into two main classes: cysteine proteases and metalloproteases. The cysteine proteases comprise ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), Machado-Josephin domain proteases (MJDs) and ovarian tumour proteases (OTU). The metalloprotease group contains only the Jab1/Mov34/Mpr1 Pad1 N-terminal+ (MPN+) (JAMM) domain proteases.

Leucyl/cystinyl aminopeptidase, also known as cystinyl aminopeptidase (CAP), insulin-regulated aminopeptidase (IRAP), human placental leucine aminopeptidase (PLAP), oxytocinase, and vasopressinase, is an enzyme of the aminopeptidase group that in humans is encoded by the LNPEP gene.

Carboxypeptidase E (CPE), also known as carboxypeptidase H (CPH) and enkephalin convertase, is an enzyme that in humans is encoded by the CPE gene. This enzyme catalyzes the release of C-terminal arginine or lysine residues from polypeptides.

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.

Methionine aminopeptidase 2 is an enzyme that in humans is encoded by the METAP2 gene.
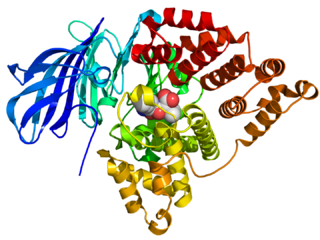
Leukotriene A4 hydrolase, also known as LTA4H is a human gene. The protein encoded by this gene is a bifunctional enzyme which converts leukotriene A4 to leukotriene B4 and acts as an aminopeptidase.

A Disintegrin and metalloproteinase domain-containing protein 10, also known as ADAM10 or CDw156 or CD156c is a protein that in humans is encoded by the ADAM10 gene.
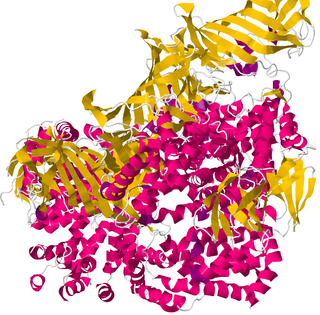
Type 1 tumor necrosis factor receptor shedding aminopeptidase regulator, also known as endoplasmic reticulum aminopeptidase 1 (ARTS-1), is a protein which in humans is encoded by the ARTS-1 gene.

Meprin A subunit alpha also known as endopeptidase-2 or PABA peptide hydrolase is the alpha subunit of the meprin A enzyme that in humans is encoded by the MEP1A gene. The MEP1A locus is on chromosome 6p in humans and on chromosome 17 in mice.

Meprin A subunit beta is a protein that in humans is encoded by the MEP1B gene.
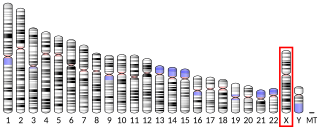
Xaa-Pro aminopeptidase 2 is an enzyme that in humans is encoded by the XPNPEP2 gene.
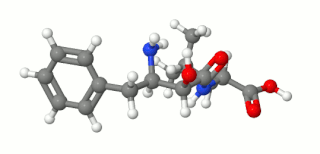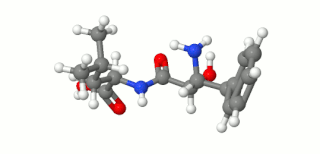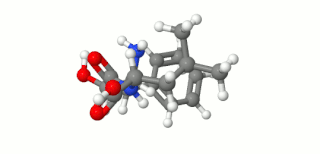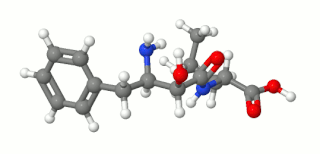
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
Aminopeptidase B is an enzyme. This enzyme catalyses the following chemical reaction
Lysine carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction:



















