Acrylonitrile is an organic compound with the formula CH2CHCN and the structure H2C=CH−C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group linked to a nitrile. It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses.
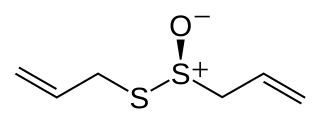
Allicin is an organosulfur compound obtained from garlic. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is an antifeedant, i.e. the defense mechanism against attacks by pests on the garlic plant.
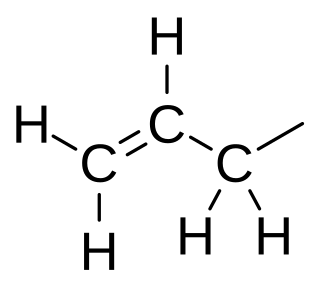
In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.
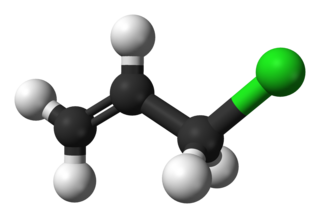
Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.

Cyclohexanol is the organic compound with the formula HOCH(CH2)5. The molecule is related to cyclohexane by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid with a camphor-like odor, which, when very pure, melts near room temperature. Millions of tonnes are produced annually, mainly as a precursor to nylon.
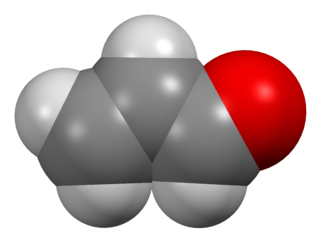
Acrolein is the simplest unsaturated aldehyde. It is a colorless liquid with a foul and acrid aroma. The smell of burnt fat is caused by glycerol in the burning fat breaking down into acrolein. It is produced industrially from propene and mainly used as a biocide and a building block to other chemical compounds, such as the amino acid methionine.

Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Millions of tonnes are produced annually, mainly as a precursor to nylon.
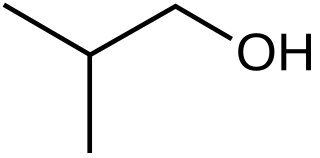
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
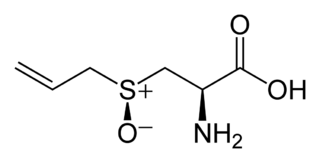
Alliin is a sulfoxide that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin and other thiosulfinates in garlic are unstable and form a number of other compounds, such as diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DAT), dithiins and ajoene. Garlic powder is not a source of alliin, nor is fresh garlic upon maceration, since the enzymatic conversion to allicin takes place in the order of seconds.
Diallyl disulfide is an organosulfur compound derived from garlic and a few other plants in the genus Allium. Along with diallyl trisulfide and diallyl tetrasulfide, it is one of the principal components of the distilled oil of garlic. It is a yellowish liquid which is insoluble in water and has a strong garlic odor. It is produced during the decomposition of allicin, which is released upon crushing garlic and other plants of the family Alliaceae. Diallyl disulfide has many of the health benefits of garlic, but it is also an allergen causing garlic allergy. Highly diluted, it is used as a flavoring in food. It decomposes in the human body into other compounds such as allyl methyl sulfide.

Epichlorohydrin is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.

Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, perfumes and other organic compounds. Allyl bromide is a colorless liquid, although commercial samples appear yellow or brown. It is an irritant and a potentially dangerous alkylating agent. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.
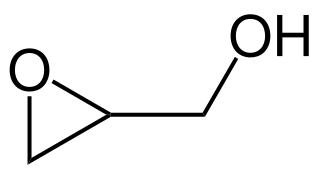
Glycidol is an organic compound with the formula HOCH2CHOCH2. The molecule contains both epoxide and alcohol functional groups. Being simple to make and bifunctional, it has a variety of industrial uses. The compound is a colorless, slightly viscous liquid that is slightly unstable and is not often encountered in pure form.
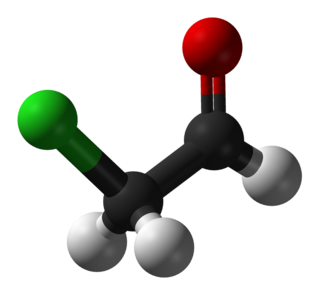
Chloroacetaldehyde is an organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hemiacetal (ClCH2CH(OH))2O.

Allyl acetate is an organic compound with formula C3H5OC(O)CH3. This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate. It is the acetate ester of allyl alcohol.
Chester J. Cavallito was an American organic chemist. He was particularly known for his work on the chemistry of garlic. Beginning in 1944, with his colleagues, he reported on the isolation from crushed garlic, synthesis and antibiotic activity of a compound he named allicin. Cavallito established that allicin was a member of a class of organosulfur compounds known as thiosulfinates. He also synthesized and reported on the chemical and biological properties of a series of thiosulfinates related to allicin.

Allyl glycidyl ether is an organic compound used in adhesives and sealants and as a monomer for polymerization reactions. It is formally the condensation product of allyl alcohol and glycidol via an ether linkage. Because it contains both an alkene and an epoxide group, either group can be reacted selectively to yield a product where the other functional group remains intact for future reactions.























