Volume 31, Number 1—January 2025
Research Letter
Spread of Antifungal-Resistant Trichophyton indotineae, United Kingdom, 2017–2024
Abstract
We describe 157 cases of Trichophyton indotineae infection in the United Kingdom, mostly in patients linked to southern Asia. T. indotineae is spreading in the United Kingdom and accounts for 38% of dermatophyte isolates referred to the UK National Mycology Reference Laboratory. Clinicians should suspect T. indotineae in tinea corporis cases.
Outbreaks of superficial skin infections caused by the emergent dermatophyte Trichophyton indotineae (Trichophyton mentagrophytes genotype VIII) were reported in southern Asia starting in 2014 (1–4). Typically, T. indotineae infections initially involve the groin (tinea cruris) and respond poorly to treatment, resulting in widespread lesions affecting multiple body sites. Many isolates exhibit in vitro resistance to terbinafine, and most infections are clinically resistant to that drug (1–5). Infections spread easily from person to person (1–8), and some reports suggest sexual transmission (9).
T. indotineae is endemic across Asia, but cases have been reported worldwide (4), including in Europe (5–7), Canada (8), and the United States (9). Mounting evidence suggests infection acquisition and transmission outside original areas of endemicity (5,7,9,10). Occasional cases of T. indotineae infection have been reported from the United Kingdom (10). We describe all cases of T. indotineae identified at the UK National Mycology Reference Laboratory (MRL) during a 7-year period.
We reviewed laboratory records from August 2017–July 2024 for dermatophytes identified as T. indotineae. When available, we extracted clinical and epidemiologic data from requisition forms. Dermatophyte identification was determined by whole-genome sequencing (WGS) or internal transcribed spacer sequencing, combined with phenotypic identification (Appendix Table). Isolates received after 2021 were identified using phenotypic features alone. A key defining microscopic feature was abundant fusiform to clavate, thin smooth-walled macroconidia with an acute apical tip, as well as other macroscopic and microscopic characteristics (Appendix Figure 1). We performed susceptibility testing by broth microdilution according to Clinical and Laboratory Standards Institute standards (Appendix). In the absence of an established clinical breakpoint for terbinafine, we used an MIC of >0.5 mg/L to identify non–wild-type isolates.
The first WGS-confirmed case we noted was from October 2018. In nearly half (42.7%, 67/157) of identified cases, the groin, buttocks, and thighs were directly involved, and neighboring body sites (abdomen and back) were implicated in another 18 cases (Table). Most (84.7%) patients had links to endemic areas, including South Asian ethnic background (n = 97), recent travel to the Indian subcontinent or Middle East (n = 41), or both (n = 36). Household spread was noted in 5 cases (Appendix Table).
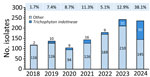
Before 2023, most (27/36) cases were identified in London, which was the most affected city according to total case numbers. Since 2023, increasing numbers of cases were found in an additional 27 cities in the United Kingdom and Ireland, and isolate numbers outside London exceed those in London (Appendix Figure 3). From 2018 to 2019, the prevalence of T. indotineae in the United Kingdom increased from 2% to 7% of all dermatophyte isolates referred to the MRL. This prevalence remained largely stable during 2019–2023 (range 5%–12%). Of note, T. indotineae comprised 38% of all dermatophyte isolates received by the MRL in 2024 up to July (Figure).
Antifungal susceptibility data for terbinafine were available for 124/157 isolates, and in vitro resistance (MIC >0.5 mg/L) was documented in 92/124 (74.2%) cases, in keeping with previous reports (1,2,4,5). Of the 108 isolates in our study, 14% displayed MICs >0.5 mg/L to itraconazole; however, a breakpoint for itraconazole with T. indotineae is lacking. Fifty (31.8%) of 157 cases had documented treatment failure, 34 (21.7%) cases had terbinafine failure, and 7 (4.5%) cases had poor response to itraconazole.
In this study, London had the highest caseloads before 2023, likely because of absolute population numbers, comprehensive travel links to the Asian subcontinent through major London airports, and enhanced access to private dermatology clinics. The largely stable prevalence from 2019 through 2023 is probably because of COVID-19 prevention measures, which reduced population mixing and subsequent spread of T. indotineae. Our findings suggest that infections were acquired either directly in southern Asia and imported into the United Kingdom or from contacts with recent travel to such areas.
The first limitation of this study is underestimation of T. indotineae prevalence because of limited awareness among medical practitioners and microbiology laboratorians, likely misidentifications in routine laboratories, lack of commercial methods for rapid and accurate identification, and difficulties in obtaining skin scrapings from patients impeding laboratory identification of causative agent. Second, probable regional differences exist in awareness and identification capacity driven by regional prevalence and likelihood of prior encounter. Third, we do not have clinical information on dose or duration of terbinafine therapy for most patients with reported treatment failures; thus, we are unable to link treatment failure to elevated MIC values. Finally, only a proportion of T. indotineae isolates had genetic confirmation of identity. Despite our confidence in our methods, the identification of some cases by phenotypic methods alone could lead to some misidentification of species within the T. mentagrophytes species complex.
In conclusion, we show that T. indotineae was introduced into the United Kingdom from endemic areas and is spreading substantially. On the basis of current trends, we predict T. indotineae will rapidly become the predominant cause of tinea corporis in the United Kingdom. Clinicians and microbiology laboratorians should recognize this fungus as a predominant cause of tinea corporis.
Dr. Abdolrasouli is a clinical scientist in medical mycology at King’s College Hospital, London, United Kingdom. His primary research interests include emerging pathogens, antifungal resistance, and laboratory diagnosis of fungal infections.
Acknowledgment
We thank Elizabeth Johnson for her interest in this work. We are also grateful to Johanna Rhodes for analyzing the whole-genome sequencing data, Daniel Kibbey for help with LIMS database searches, Sue McLachlan for assistance with isolate identification, and Sue McLachlan, Cheryl Yung, and Patricia Coll-Gutierrez for performing antifungal drug susceptibility testing of Trichophyton indotineae isolates.
References
- Singh A, Masih A, Monroy-Nieto J, Singh PK, Bowers J, Travis J, et al. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: Genomic insights and resistance profile. Fungal Genet Biol. 2019;133:
103266 . DOIPubMedGoogle Scholar - Kano R, Kimura U, Kakurai M, Hiruma J, Kamata H, Suga Y, et al. Trichophyton indotineae sp. nov.: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185:947–58. DOIPubMedGoogle Scholar
- Chowdhary A, Singh A, Kaur A, Khurana A. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: A new challenge in the management of dermatophytosis. PLoS Pathog. 2022;18:
e1010795 . DOIPubMedGoogle Scholar - Dellière S, Jabet A, Abdolrasouli A. Current and emerging issues in dermatophyte infections. PLoS Pathog. 2024;20:
e1012258 . DOIPubMedGoogle Scholar - Brasch J, Gräser Y, Beck-Jendroscheck V, Voss K, Torz K, Walther G, et al. “Indian” strains of Trichophyton mentagrophytes with reduced itraconazole susceptibility in Germany. J Dtsch Dermatol Ges. 2021;19:1723–7. DOIPubMedGoogle Scholar
- Dellière S, Joannard B, Benderdouche M, Mingui A, Gits-Muselli M, Hamane S, et al. Emergence of difficult-to-treat tinea corporis caused by Trichophyton mentagrophytes complex isolates, Paris, France. Emerg Infect Dis. 2022;28:224–8. DOIPubMedGoogle Scholar
- Siopi M, Efstathiou I, Theodoropoulos K, Pournaras S, Meletiadis J. Molecular epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: emergence of terbinafine-resistant Trichophyton mentagrophytes type VIII locally and globally. J Fungi (Basel). 2021;7:419. DOIPubMedGoogle Scholar
- Posso-De Los Rios CJ, Tadros E, Summerbell RC, Scott JA. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J Cutan Med Surg. 2022;26:371–6. DOIPubMedGoogle Scholar
- Spivack S, Gold JAW, Lockhart SR, Anand P, Quilter LAS, Smith DJ, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807–9. DOIPubMedGoogle Scholar
- Abdolrasouli A, Borman AM, Johnson EM, Hay RJ, Arias M. Terbinafine-resistant Trichophyton indotineae causing extensive dermatophytosis in a returning traveller, London, UK. Clin Exp Dermatol. 2024;49:635–7. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: December 17, 2024
Table of Contents – Volume 31, Number 1—January 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Correspondence: Andrew M. Borman, Mycology Reference Laboratory, UK Health Security Agency, Science Quarter, Southmead Hospital, Bristol BS10 5NB, UK
Top