Volume 28, Number 8—August 2022
Dispatch
Anthelmintic Baiting of Foxes against Echinococcus multilocularis in Small Public Area, Japan
Abstract
We distributed anthelmintic baits on a university campus in Japan inhabited by foxes infected with Echinococcus multilocularis to design an effective baiting protocol for small public areas. High-density baiting can reduce the risk for human exposure to the parasite to near zero. However, monthly baiting is recommended to maintain this effect.
Alveolar echinococcosis is a potentially fatal disease caused by the larvae of the Echinococcus multilocularis tapeworm, which is widely distributed in the Northern Hemisphere (1). This parasite primarily depends on red foxes as definitive hosts, along with small mammals (mainly Myodes rufocanus gray-backed voles) as intermediate hosts in Japan (2). Human infection occurs by accidental ingestion of the parasite eggs excreted through the feces of definitive hosts.
Field trials aimed at reducing the rate of E. multilocularis infection in foxes through the distribution of praziquantel-containing baits have been conducted in Europe and Japan (3–7). These studies showed that anthelmintic baiting over a large area effectively reduces the infection rate in foxes; however, in most cases, eradicating the parasite from the area is difficult. Urban fox populations have increased in many countries in recent decades. In Hokkaido, Japan, foxes invade and breed on smaller spatial scales, such as university campuses and zoos in urban areas (8). Several deaths in zoo animals infected with echinococcosis have also been reported (9). Reducing the risk for infection among workers, students or visitors, and zoo animals has become an important issue for facility managers. Anthelmintic baiting may be an efficient measure against echinococcosis in such areas with many users on a small spatial scale. However, the effect of baiting on such small public areas has not been widely examined (10).
We conducted this study to provide a basic dataset for designing an effective baiting protocol for small public areas. We investigated the effect of high-density baiting on contamination by E. multilocularis eggs on a university campus in Japan.
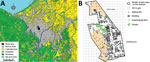
The study was conducted on the Hokkaido University campus (an area of 1.8 km2) in an urban area of Sapporo, Japan (Figure 1, panel A). We evenly distributed anthelmintic baits manually by using 100-m grids on a map (Figure 1, panel B). We structured bait distribution into 2 phases. In phase 1 (August 2014–early July 2016), we distributed 100 baits/km2 monthly across the campus during the summer and fall of 2014 and 2015. In phase 2 (late July 2016–December 2018), we distributed baits monthly throughout the year. We excluded the building area (Figure 1, panel B) from baiting in this phase because the bait consumption and frequency of foxes in the camera survey in this area were relatively low compared with the farm area in phase 1. We reduced the baiting area to ≈70% of the campus (an area of 1.3 km2), and the density of baits on the campus decreased to ≈70/km2. These baiting densities in this study are higher than those used in previous studies. We prepared anthelmintic baits for this study by mixing praziquantel with fishmeal and 2 types of edible fats, which we formed into pellets containing 50 mg praziquantel each (11).
To determine the effect of baiting, we detected E. multilocularis eggs in fox feces, we collected fox fecal samples on campus mainly during the snowless season (Figure 2). We examined parasite eggs in all fecal samples by using a sugar flotation method with 1 g of feces (12), and then we molecularly analyzed the species of detected taeniid egg by using PCR/sequencing of the cytochrome c oxidase subunit 1 and nuclear U1 spliceosomal RNA genes (13). We also examined fecal samples collected in phase 2 for copro-DNA derived from the body of an adult E. multilocularis worm by using 3 g of feces (14). We recorded the number of fox fecal samples collected on the campus and presence or absence of E. multilocularis eggs or DNA in the samples (Figure 2). Before the first bait campaign, 53.4% (31/58) of the collected feces contained eggs. In phase 1, we collected 144 fecal samples, 2.1% (3/144) of which were egg-positive. We identified all detected eggs as E. multilocularis by analyzing the cytochrome c oxidase subunit 1 and nuclear U1 spliceosomal RNA gene sequences. We found no egg-positive feces during the baiting period (Figure 2). In phase 2, none of the 282 fecal samples collected during September 2016–October 2018 contained eggs (Table). However, we detected E. multilocularis–specific DNA by using the copro-DNA test on 5 fecal samples collected in phase 2 (Figure 2).
We investigated the prevalence of E. multilocularis larvae in intermediate hosts on the campus. We set 150–250 traps (H.B. Sherman Traps Inc., https://www.shermantraps.com) for 3 consecutive days in the spring, summer, and fall seasons of 2014–2018, except for the spring of 2014 (Figure 2). We dissected all captured mammals and examined them macroscopically for lesions in the liver and other organs. We investigated lesions for E. multilocularis metacestode tissues by examining morphologic features. We determined the age of M. rufocanus voles, the most important intermediate host in Hokkaido (2), by examining the shape and root ratio of the molars (15). In total, we captured 649 small mammals of 6 species on the campus (Appendix Table). Seven of the 508 M. rufocanus voles were infected with E. multilocularis. The age of all M. rufocanus voles ranged from 20 days to 16 months. Of these, 6.8% were older than 12 months. The ages (+ SD of the z score) of the 7 infected voles were 51 + 20 days and 5 + 1.0 (2 individual voles), 6 + 1.5, 12 + 3.0, 13 + 3.8, and 14 + 3.8 months. Judging from their ages, we determined that the lifespan of all infected voles included the nonbaiting period (Figure 2). None of the 286 voles born in phase 2 were infected. These results show that if egg-positive fox feces are present during the nonbaiting period, voles can be infected with E. multilocularis worms and remain a source for infection of foxes for a year or more.
Although the egg-positive rate of fecal samples is not equivalent to the infection rate in foxes, this rate directly represents the risk for exposure to the parasite eggs when university staff and students come into contact with the feces on campus. The goal of baiting on the campus is not to reduce the infection rate in foxes, but to reduce the egg-positive rate to near zero to prevent human infection with E. multilocularis tapeworms within the campus.
In this study, high-density, monthly baiting nearly eradicated the parasite eggs in a campus for >2 years. The effectiveness of high-density baiting has also been demonstrated in Europe, although the evaluation methods were different (3,10). In contrast, when the baiting was suspended, egg-positive feces were found again in 6–7 months, possibly because of the long lifespan of the intermediate host. Even after monthly baiting for 22 months, longer than the generation time of voles, DNA-positive feces were found in June 2018, possibly because of migrating foxes. These results suggest that preventing reinfection of foxes is difficult, even in a small area. However, even if reinfection occurs, monthly baiting will probably eliminate the parasites before the foxes excrete the parasite eggs, because the monthly interval is approximately the same as the prepatent period for E. multilocularis tapeworms. Eradicating E. multilocularis tapeworms from an area is difficult, but eradicating the parasite eggs may be possible.
These findings are subject to limitations because results may not be completely generalizable. Further studies are needed to identify individual feces using genetic analysis to achieve a more detailed understanding of the mechanism of small area baiting. In summary, high-density, monthly baiting is effective for preventing human infection with E. multilocularis tapeworms within small public areas.
Mr. Uraguchi studied forestry and zoology at Hokkaido University, Japan, and is currently a senior researcher of Hokkaido Institute of Public Health. His main research interests are animal ecology, medical zoology, and urban ecology.
Acknowledgments
We thank Takashi Saitoh for his valuable comments on the manuscript. We also thank the experimental farm staff of Hokkaido University for providing important information about foxes on campus.
This research was supported by JSPS KAKENHI (grant no. JP20K06402).
References
- World Health Organization/World Health Organization for Animal Health. Geographic distribution and prevalence. In: Eckert J, Gemmell MA, Meslin FX, Pawłowski ZS, editors. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: World Health Organization/World Health Organization for Animal Health; 2001. p. 101–43 [cited 2022 May 1]. https://apps.who.int/iris/handle/10665/42427
- Ito A, Romig T, Takahashi K. Perspective on control options for Echinococcus multilocularis with particular reference to Japan. Parasitology. 2003;127(Suppl):S159–72. DOIPubMedGoogle Scholar
- König A, Romig T, Holzhofer E. Effective long-term control of Echinococcus multilocularis in a mixed rural-urban area in southern Germany. PLoS One. 2019;14:
e0214993 . DOIPubMedGoogle Scholar - Tackmann K, Löschner U, Mix H, Staubach C, Thulke HH, Ziller M, et al. A field study to control Echinococcus multilocularis-infections of the red fox (Vulpes vulpes) in an endemic focus. Epidemiol Infect. 2001;127:577–87. DOIPubMedGoogle Scholar
- Romig T, Bilger B, Dinkel A, Merli M, Thoma D, Will R, et al. Impact of praziquantel baiting on intestinal helminths of foxes in southwestern Germany. Helminthologia. 2007;44:137–44. DOIGoogle Scholar
- Tsukada H, Hamazaki K, Ganzorig S, Iwaki T, Konno K, Lagapa JT, et al. Potential remedy against Echinococcus multilocularis in wild red foxes using baits with anthelmintic distributed around fox breeding dens in Hokkaido, Japan. Parasitology. 2002;125:119–29. DOIPubMedGoogle Scholar
- Takahashi K, Uraguchi K, Hatakeyama H, Giraudoux P, Romig T. Efficacy of anthelmintic baiting of foxes against Echinococcus multilocularis in northern Japan. Vet Parasitol. 2013;198:122–6. DOIPubMedGoogle Scholar
- Uraguchi K. Red fox. In: Masuda R, editor. Carnivores in Japan: mammals at the top of the ecosystem [in Japanese]. Tokyo: University of Tokyo Pres; 2018. p. 67–88.
- Yamano K, Kouguchi H, Uraguchi K, Mukai T, Shibata C, Yamamoto H, et al. First detection of Echinococcus multilocularis infection in two species of nonhuman primates raised in a zoo: a fatal case in Cercopithecus diana and a strongly suspected case of spontaneous recovery in Macaca nigra. Parasitol Int. 2014;63:621–6. DOIPubMedGoogle Scholar
- Hegglin D, Deplazes P. Control strategy for Echinococcus multilocularis. Emerg Infect Dis. 2008;14:1626–8. DOIPubMedGoogle Scholar
- Takahashi K, Uraguchi K, Abe S, Hirakawa H. Making acceptable bait by red foxes [in Japanese]. Report of the Hokkaido Institute of Public Health. 2010;60:81–2.
- Ito S. Modified Wisconsin sugar centrifugal-flotation technique for nematode eggs in bovine feces [in Japanese.]. Nippon Juishikai Zasshi. 1980;33:424–9. DOIGoogle Scholar
- Irie T, Mukai T, Yagi K. Echinococcus multilocularis surveillance using copro-DNA and egg examination of shelter dogs from an endemic area in Hokkaido, Japan. Vector Borne Zoonotic Dis. 2018;18:390–2. DOIPubMedGoogle Scholar
- Irie T, Ito T, Kouguchi H, Yamano K, Uraguchi K, Yagi K, et al. Diagnosis of canine Echinococcus multilocularis infections by copro-DNA tests: comparison of DNA extraction techniques and evaluation of diagnostic deworming. Parasitol Res. 2017;116:2139–44. DOIPubMedGoogle Scholar
- Abe H. Age determination of Clethrionomys rufocanus bedfordiae (THOMAS) [in Japanese.]. Jap J Ecol. 1976;26:221–7.
Figures
Table
Cite This ArticleOriginal Publication Date: July 08, 2022
1These authors contributed equally to this article.
2Current affiliation: University of Miyazaki, Miyazaki, Japan.
3Current affiliation: Hokkaido University, Sapporo, Japan.
Table of Contents – Volume 28, Number 8—August 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Kohji Uraguchi, Department of Infectious Diseases, Hokkaido Institute of Public Health, N19 W12, Sapporo 060-0819, Japan
Top