Volume 27, Number 7—July 2021
Research Letter
Anthemosoma garnhami in an HIV-Infected Man from Zimbabwe Living in South Africa
Abstract
An HIV-positive man from Zimbabwe living in South Africa sought treatment for multiple clinical signs, including fever, weight loss, anemia, and splenomegaly. We identified in his blood an African rodent piroplasm, Anthemosoma garnhami, related to Babesia species. This finding extends the known geographic and host range of A. garnhami.
A 24-year-old man from Zimbabwe who had been living in East London, South Africa, for 13 years attended a primary health care clinic in East London complaining of a 3-month period of generalized body pains, drenching night sweats, and weight loss. He had no notable previous medical history. The attending nurse diagnosed HIV infection by rapid test, collected sputum for an Xpert MTB/RIF test (Cepheid, https://www.cepheid.com), and requested blood screening as preparation before initiating combination antiretroviral therapy. Malaria-like objects found on the blood smear prompted referral for specialist opinion at Cecilia Makiwane Hospital in Mdantsane, South Africa. This case report was approved by the Human Research Committee of the Faculty of Health Sciences, Walter Sisulu University, Mthatha, South Africa (protocol no. 126/2020). The patient granted written informed consent for publication of the case report.
The patient shared a house with another adult (no animals) and worked as a construction laborer. Four months before seeking treatment, he returned from a 2-month home visit to Masvingo Province in Zimbabwe. He did not recall tick bites but reported that goats and cattle lived in the village he visited.
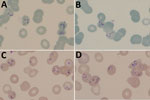
At hospital admission, the patient was wasted (40 kg), generally weak, afebrile, and markedly pale; he had oral candidiasis. His enlarged, smooth, nontender spleen was palpable to ≈10 cm below the costal margin in the midclavicular line. No other findings were remarkable. Laboratory results (Table) showed evidence of likely hypersplenism-related pancytopenia, hemolysis, mildly raised transaminases, and advanced HIV infection. The abnormal blood smear showed intraerythrocytic parasites, initially thought to be malarial. However, concurrent rapid malaria antigen tests were negative, and the smears and whole blood sample were sent to a national parasitology reference laboratory for further assessment. On the basis of microscopic examination of Giemsa-stained blood smears (Figure), we diagnosed babesiosis accompanied by hemolytic anemia.
We started the patient on a 10-day course of oral clindamycin and quinine (each 600 mg every 8 h). Blood transfusion was not needed. After 2 weeks, all symptoms improved markedly; splenomegaly was reduced to 5 cm below the costal margin and hemoglobin substantially improved. We subsequently initiated tenofovir, emtricitabine/efavirenz combination antiretroviral therapy, and trimethoprim/sulfamethoxazole prophylaxis; the patient responded well in the hospital antiretroviral unit.
DNA extracted from anticoagulated whole blood tested negative for Plasmodium spp. by multiplex real-time PCR for malaria. We used a nested conventional PCR assay for the Babesia species 18S RNA gene (1) and applied bidirectional Sanger sequencing to the 400-bp product, showing sequences shared across members of order Piroplasmida. We used PCR with 18S RNA universal primers to refine this result. (2). The ≈1,700 bp product sequence (GenBank accession no. MW276138) had 99.15% identity and a subsequent sequence (accession no. MW276139) from a recrudescence, 99.03% identity with the murine piroplasm Anthemosoma garnhami (accession no. MH093637.1; Appendix Figure).
The patient returned for treatment 14 months later. He had not traveled outside of South Africa since his initial treatment. He was again pale and had an enlarged spleen. His hemoglobin was 59 g/L, and we again observed intraerythrocytic piroplasms on the blood smear. His CD4 count was now 195 cells/mm3 and HIV viral load 304 copies/mL. He was admitted for intravenous clindamycin and oral quinine (each 600 mg every 8 h) as part of a 6-week treatment plan. The patient responded well clinically and hematologically to treatment (Table).
A. garnhami is an erythrocytic murine parasite, first described in spiny mice (Acomys percivali) in Ethiopia in 1969 (3). Because it shares characteristics with Haemosporidia and Piroplasmida, its classification was long debated, but on the basis of ribosomal RNA analysis of archived A. garnhami samples, it was finally assigned to the piroplasms, as the sole species of the family Anthemosomatidae (3). The parasite was identified again in 2 different rodent species in Namibia (4,5). Ixodid ticks serve as vectors of piroplasms and therefore are likely vectors for A. garnhami; experiments failed to demonstrate transmission by several tick and mosquito species (3). A. garnhami is closely related to the babesids in the Piroplasmida order, hence the similar microscopic appearance and the good clinical response in this case to clindamycin and quinine, drugs used to treat Babesia spp. Babesiosis in immunocompromised patients, including those with HIV, is more severe and more likely to recur (6). The recrudescing clinical course of this A. garnhami infection was probably exacerbated by the patient’s advanced HIV disease.
Our report establishes a likely epizootologic similarity between A. garnhami and Babesia spp., suggesting the potential for A. garnhami to cause zoonotic infections in humans. Although babesiosis in domestic animals is common in Africa and B. microti has been found in nonhuman primates in East Africa (7), only single reports from southern Africa (8) and Equatorial Guinea (9) have described human Babesia spp. infections. The conjunction of high concentrations of ticks, animals, malaria, and HIV-infected humans in Africa make it possible for piroplasm infections to be misdiagnosed as malaria, which poses potentially serious clinical consequences for immunocompromised patients.
Dr. Stead is the head of the Division of Infectious Diseases in the Departments of Medicine at Frere and Cecilia Makiwane hospitals and senior lecturer in the Department of Medicine at the Walter Sisulu University Health Sciences Faculty. His primary research interests are tuberculosis diagnostics, HIV-related opportunistic infections, and severe acute respiratory syndrome coronavirus 2 healthcare worker infections.
Acknowledgment
We are grateful to Michelle Bosman for the initial laboratory investigations and to Marinda Oosthuizen, Ilse Vorster, and Milana Troski for the gift of Babesia spp. DNA extracts.
References
- Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, et al. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol. 2001;39:2178–83. DOIPubMedGoogle Scholar
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–9. DOIPubMedGoogle Scholar
- Chavatte JM, Karadjian G, Landau I. Half a century after its discovery, new insights on Anthemosoma garnhami (Sporozoa, Piroplasmida): morphology, molecular characterisation and phylogenetic position. Parasitol Res. 2018;117:3917–25. DOIPubMedGoogle Scholar
- Gunders AE. Anthemosoma sp. isolated from Aethomys namaquensis in Namibia [conference abstract]. S Afr J Sci. 1985;81:48.
- Gunders AE. Anthemosoma sp. from Thallomys paedulcus (Sundevall, 1847) from Namibia—a new host record [conference abstract]. S Afr J Sci. 1986;82:652.
- Maamun JM, Suleman MA, Akinyi M, Ozwara H, Kariuki T, Carlsson HE. Prevalence of Babesia microti in free-ranging baboons and African green monkeys. J Parasitol. 2011;97:63–7. DOIPubMedGoogle Scholar
- Bush JB, Isaäcson M, Mohamed AS, Potgieter FT, de Waal DT. Human babesiosis—a preliminary report of 2 suspected cases in South Africa. S Afr Med J. 1990;78:699.PubMedGoogle Scholar
- Arsuaga M, González LM, Padial ES, Dinkessa AW, Sevilla E, Trigo E, et al. Misdiagnosis of babesiosis as malaria, Equatorial Guinea, 2014. Emerg Infect Dis. 2018;24:1588–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: June 11, 2021
Table of Contents – Volume 27, Number 7—July 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
John Frean, National Institute for Communicable Diseases, Parasitology Reference Laboratory, P/Bag X4, Sandringham, Johannesburg 2131, South Africa
Top