Volume 23, Number 9—September 2017
CME ACTIVITY - Research
Epidemiology of Salmonella enterica Serotype Dublin Infections among Humans, United States, 1968–2013
Introduction

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; and (4) view/print certificate.
Release date: August 16, 2017; Expiration date: August 16, 2018
Learning Objectives
Upon completion of this activity, participants will be able to:
• Evaluate the incidence and demographics of Salmonella serotype Dublin human infections, based on an analysis of US national surveillance data
• Distinguish the clinical severity of Salmonella serotype Dublin human infections, based on an analysis of US national surveillance data
• Assess the antimicrobial resistance of Salmonella serotype Dublin human infections, based on an analysis of US national surveillance data.
CME Editor
P. Lynne Stockton Taylor, VMD, MS, ELS(D), Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: P. Lynne Stockton Taylor, VMD, MS, ELS(D), has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed the following relevant financial relationships: owns stock, stock options, or bonds from Alnylam; Biogen; Pfizer.
Authors
Disclosures: R. Reid Harvey, DVM, MPH; Cindy R. Friedman, MD; Stacy M. Crim, MPH, BS; Michael Judd, MPH; Kelly A. Barrett, MPH; Beth Tolar, MS; Jason P. Folster, PhD; Patricia M. Griffin, MD; and Allison C. Brown PhD, MPH, have disclosed no relevant financial relationships.
Abstract
Salmonella enterica serotype Dublin is a cattle-adapted bacterium that typically causes bloodstream infections in humans. To summarize demographic, clinical, and antimicrobial drug resistance characteristics of human infections with this organism in the United States, we analyzed data for 1968–2013 from 5 US surveillance systems. During this period, the incidence rate for infection with Salmonella Dublin increased more than that for infection with other Salmonella. Data from 1 system (FoodNet) showed that a higher percentage of persons with Salmonella Dublin infection were hospitalized and died during 2005−2013 (78% hospitalized, 4.2% died) than during 1996–2004 (68% hospitalized, 2.7% died). Susceptibility data showed that a higher percentage of isolates were resistant to >7 classes of antimicrobial drugs during 2005–2013 (50.8%) than during 1996–2004 (2.4%).
Salmonella Dublin is a zoonotic Salmonella enterica serotype that in recent years has increased in infection incidence, antimicrobial drug resistance, and illness clinical severity. The Centers for Disease Control and Prevention (CDC) estimates that each year in the United States, Salmonella enterica causes 1.2 million infections, 24,000 hospitalizations, and 450 deaths (1). Although >2,500 serotypes of Salmonella exist (2), only ≈50 serotypes are regularly isolated from humans. Illnesses caused by nontyphoidal Salmonella are often self-limiting and require no antimicrobial drug therapy, but for patients with invasive infections, treatment is critical. Antimicrobial drug–resistant strains of Salmonella are associated with more severe illness and are more likely to result in bloodstream infection, hospitalization, and death than are illnesses caused by drug-susceptible strains (3,4). According to surveillance data from the National Antimicrobial Resistance Monitoring System (NARMS), the proportion of resistant isolates is higher among S. enterica serotype Dublin than among other serotypes (5).
Unlike most nontyphoidal Salmonella serotypes, which affect a broad spectrum of unrelated host species, Salmonella Dublin is a cattle-adapted serotype (6). The most comprehensive analysis of cases of Salmonella Dublin infection was published in 1982 and demonstrated that this serotype causes rare but severe disease in humans (i.e., bloodstream infection) that often requires antimicrobial drug therapy (7). Using available data across various CDC surveillance systems, we analyzed the epidemiology of human infections with Salmonella Dublin in the United States, including antimicrobial drug resistance, and compared it with that of other Salmonella serotypes.
Data Sources
Laboratory-based Enteric Disease Surveillance, 1968–2013
Begun in 1968, the CDC Laboratory-based Enteric Disease Surveillance system (LEDS) collects serotype and demographic data for every Salmonella isolate obtained from a human and submitted to US state and territorial public health laboratories. We used LEDS data to estimate national incidence rates (no. cases/100,000 population, using US census population estimates) of reported Salmonella Dublin and other nontyphoidal Salmonella serotypes. We excluded all typhoidal serotypes: Typhi, Paratyphi A, Paratyphi B (L[+] tartrate-negative), and Paratyphi C. We defined other nontyphoidal Salmonella as serotypes other than Salmonella Dublin (hereafter called other Salmonella). We also used LEDS data to evaluate differences in proportions of patients by race, ethnicity, and home state, infected with Salmonella Dublin and other Salmonella serotypes.
Foodborne Diseases Active Surveillance Network, 1996–2013
Since 1996, the Foodborne Diseases Active Surveillance Network (FoodNet) has conducted active, population-based surveillance for culture-confirmed cases of infection caused by 9 pathogens, including Salmonella, transmitted commonly through food in the United States. FoodNet is a collaboration of CDC, 10 state health departments, the US Department of Agriculture Food Safety and Inspection Service (USDA FSIS), and the US Food and Drug Administration (FDA). The FoodNet surveillance area includes 15% of the US population. For each reported case, FoodNet sites collect data on demographic characteristics, hospitalization, and outcome. Since 2004, FoodNet has also collected data on international travel (defined as travel abroad in the 7 days before illness began) and whether the case was associated with an outbreak. We used FoodNet data to compare demographics, clinical outcomes, and travel history among patients infected with Salmonella Dublin and those infected with other Salmonella serotypes.
National Molecular Subtyping Network for Foodborne Disease Surveillance, 1996–2013
Begun in 1996, the National Molecular Subtyping Network for Foodborne Disease Surveillance (PulseNet) is a national network of state and local public health laboratories and food regulatory agencies in the United States. Laboratorians upload pulsed-field gel electrophoresis patterns to PulseNet national databases. Comparison of these patterns enables identification of matches and possible outbreaks. The PulseNet database contains isolate data from human, food, environmental, and animal sources. We used PulseNet data to identify common nonhuman sources of Salmonella Dublin isolates.
Foodborne Disease Outbreak Surveillance System, 1973–2013
Since 1973, the Foodborne Disease Outbreak Surveillance System (FDOSS) has collected reports of enteric disease outbreaks transmitted by food in the United States. State and local public health agencies submit to CDC reports that include information about outbreak characteristics, food vehicles, and pathogens that caused each outbreak. We searched FDOSS data to describe the vehicles implicated in outbreaks of Salmonella Dublin infections.
NARMS, 1996–2013
Begun in 1996, NARMS is a collaboration among CDC, FDA, USDA, and state and local health departments. CDC asks public health laboratories that participate in LEDS to submit every 20th Salmonella isolate received from clinical laboratories to NARMS for the purpose of tracking changes in the antimicrobial susceptibility of certain enteric bacteria isolated from ill persons, retail meats, and food animals. We included NARMS data to compare antimicrobial resistance profiles (resistance to clinically important agents and the number of resistant classes) of Salmonella Dublin isolates from humans with those from other Salmonella serotypes. Susceptibility testing was conducted as previously described (8). In brief, isolates were tested for antimicrobial susceptibility by using broth microdilution (Sensititer; Trek Diagnostics, Cleveland, OH, USA) to determine the MIC for 14 antimicrobial agents (amikacin, gentamicin, streptomycin, ampicillin, amoxicillin/clavulanic acid, ceftiofur, ceftriaxone, cefoxitin, sulfamethoxazole/sulfisoxazole, trimethoprim/sulfamethoxazole, chloramphenicol, ciprofloxacin, nalidixic acid, and tetracycline). These agents were categorized into 8 classes, as defined by Clinical and Laboratory Standards Institute guidelines. When available, Clinical and Laboratory Standards Institute interpretive criteria were used to define resistance (5). A subset of isolates that showed resistance to ceftiofur or ceftriaxone were also tested for ceftazidime susceptibility. A multidrug-resistant (MDR) isolate was defined as one resistant to >3 classes of drug. We also examined specific resistance patterns, which included isolates that were resistant to at least ampicillin, chloramphenicol, streptomycin, sulfonamide (sulfamethoxazole/sulfisoxazole), and tetracycline (ACSSuT) and isolates that were also resistant to amoxicillin/clavulanic acid and ceftriaxone (ACSSuTAuCx). We compared antimicrobial resistance patterns of Salmonella Dublin between the 2 periods 1996–2004 and 2005–2013.
Statistical Analyses
We used the Pearson χ2 test for statistical comparisons. We considered differences significant if the p value was <0.05. Statistical analyses were conducted by using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Incidence
During 1968–2013, states reported 3,903 cases of Salmonella Dublin infections to LEDS. These cases accounted for <0.25% of Salmonella infections reported. The incidence rate (no. Salmonella Dublin infections/100,000 persons) has been steadily rising since 1968 (0.0055 infections) with the exception of a distinct increase and subsequent decrease in incidence occurring throughout the 1980s, peaking in 1985 at 0.081 infections (Figure 1). The incidence rate for Salmonella Dublin infection was 7.6 times higher in 2013 (0.042 infections) than in 1968. In contrast, the incidence rate of other Salmonella infections has remained relatively stable since 1968 (9.5 infections compared with 11.2 infections in 2013). More than half (51%; 1,989/3,903) of all Salmonella Dublin infections were among California residents, including 74% (484/656) of infections during the peak in incidence from 1982 to 1985 (Figure 2). According to LEDS data, most Salmonella Dublin infections are reported from California; during 2005–2013, the 271 Salmonella Dublin infections reported from California accounted for 29% of the 943 cases reported to LEDS.
Demographics
Demographics differed markedly among those infected with Salmonella Dublin and those with other Salmonella. According to FoodNet data, 38% of Salmonella Dublin infections occurred in persons >65 years of age, compared with 11% of other Salmonella infections (p<0.01) (Table 1). The median age of Salmonella Dublin patients was 55 years; the median age of patients with other Salmonella infections was 23 years (p<0.01). A total of 7% of Salmonella Dublin infections and 28% of other Salmonella infections occurred in children <5 years of age (p<0.01); 60% of Salmonella Dublin and 48% of other Salmonella infections occurred in men (p<0.01). We found no significant difference in history of international travel between patients with Salmonella Dublin (5%; 6/101) and other Salmonella infections (9%; 4,297/46,764) (p = 0.15).
Clinical Outcomes and Severity of Disease
According to FoodNet data, Salmonella Dublin was more commonly isolated from blood (61%) than were other Salmonella (5%) (p<0.01) (Table 1). Hospitalization was reported for 75% of patients with Salmonella Dublin infection and 27% of patients with other Salmonella infections (p<0.01) (Table 1). Hospitalization lasted a median of 6 days for patients with Salmonella Dublin infection and 3 days for patients with other Salmonella infections (p<0.01). Salmonella infection resulted in death for 4% of patients with Salmonella Dublin infection and 0.5% of patients with other Salmonella infections (p<0.01).
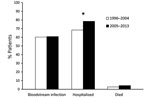
The proportion of Salmonella Dublin isolates from blood remained relatively constant during 1996–2004 (60%) and 2005–2013 (61%) (Figure 3). Hospitalization among Salmonella Dublin patients increased from 68% during 1996–2004 to 78% during 2005–2013 (p<0.05). The mortality rate increased from 2.7% during 1996–2004 to 4.2% during 2005–2013 (p = 0.57).
Sources
Food and Animals
According to the PulseNet database, 478 Salmonella Dublin isolates were obtained from food during 1999–2013. Source data for 475 foodborne isolates indicated that 473 (99%) were from beef, 1 was from cooked pork, and 1 was from chili pepper. During this same period, another 376 Salmonella Dublin isolates were obtained from animals. Of the 331 of these isolates with source data available, 328 (99%) were from cattle and 3 were from a pig, a dog, and a horse.
Outbreaks
During 1973–2013, a total of 9 Salmonella Dublin outbreaks were reported to FDOSS. These outbreaks occurred in California (5 outbreaks), Washington (2), Arkansas (1), and Wisconsin (1). Of the 9 outbreaks, 6 (67%) occurred before 1982. For each of 3 outbreaks, the foodborne vehicle was identified (raw beef, raw milk, and Mexican-style cheese).
Sporadic Illnesses
We used LEDS data to determine the proportion of all Salmonella infections reported during 2007–2012. Salmonella Dublin infection was more common in states where the sale of raw milk is legal (328 cases/100,000 persons) (9) than in states where such sale is illegal (108 cases/100,000 persons) (p<0.01).
Antimicrobial Resistance
During 1996–2013, a total of 102 clinical isolates of Salmonella Dublin were tested by NARMS (Table 2). Of these 102 isolates, 42 (41%) were pansusceptible; of the 33,415 isolates from other Salmonella, 26,552 (79%) were pansusceptible (p<0.01). Ceftriaxone resistance increased from detection in 0 of 5 isolates in 1996 to detection in 11 (92%) of 12 isolates in 2013 and was higher among Salmonella Dublin isolates (31%; 32/102) than among other Salmonella isolates (3%; 947/33,415) (p<0.01). Of the 31 ceftriaxone-resistant isolates that were also tested for ceftazidime resistance, 28 (90%) were resistant.
Multidrug resistance was found for 56 (55%) of Salmonella Dublin isolates compared with 4,013 (12%) of other Salmonella isolates (p<0.01) (Table 2). Among MDR Salmonella Dublin isolates, 84% were resistant to >5 classes of antimicrobial drugs and 57% were resistant to >7 classes; among MDR isolates of other Salmonella, 59% (p<0.01) were resistant to >5 classes and 15% (p<0.01) were resistant to >7 classes. ACSSuT resistance was found in 41% of Salmonella Dublin isolates, compared with 7% of other Salmonella isolates (p<0.01).
ACSSuTAuCx resistance was found for 28% of Salmonella Dublin isolates and 2% of other Salmonella isolates (p<0.01). Resistance to nalidixic acid was found for 6% of Salmonella Dublin isolates and 2% of other Salmonella isolates (p<0.01). Among nalidixic acid–resistant isolates, 67% of Salmonella Dublin isolates and 6% of other Salmonella isolates were also resistant to ceftriaxone (p<0.01). The proportion of Salmonella Dublin isolates resistant to antimicrobial drugs increased markedly from 1996–2004 to 2005–2013, from 29% to 79% for resistance to >1 classes (p<0.01) and from 2% to 51% for resistance to >7 antimicrobial classes (p<0.01) (Figure 4). Among resistant isolates, the median number of classes to which isolates were resistant increased from 4.5 to 7.0 (p<0.01). Resistance to ceftriaxone increased from 3% during 1996–2004 to 52% during 2005–2013 (p<0.01), and resistance to nalidixic acid increased from 0 to 10% during these same periods (p<0.05).
Decades of CDC surveillance data analyzed in this study illustrate that Salmonella Dublin more often causes bloodstream infections, hospitalizations (with longer hospital stays), and deaths than other Salmonella serotypes. Our findings support previous descriptions of Salmonella Dublin as a cattle-adapted serotype (10).
In the past decade, more than half of Salmonella Dublin infections have been resistant to >7 antimicrobial drug classes, and clinical outcomes have been more severe. The proportion of Salmonella Dublin isolates that were resistant was ≈2.7 times greater during 2005–2013 than during 1996–2004; isolates from the later period were also resistant to more antimicrobial drug classes. Multidrug resistance probably has direct clinical implications because bloodstream infections that require antimicrobial therapy tend to develop in patients with Salmonella Dublin infections (3). Most Salmonella Dublin isolates were resistant to third-generation cephalosporins (including ceftriaxone), which are often the treatment of choice for children with bloodstream infections because of the contraindication for fluoroquinolone use in children.
Clinical severity of Salmonella Dublin infections, as measured by the proportion of hospitalizations and deaths, also increased between 1996‒2004 and 2005‒2013. Our study did not directly measure the association between antimicrobial resistance and clinical severity of Salmonella Dublin infection by linking isolate data to outcome data. Nevertheless, by comparing both measures over the 2 periods, we showed that, for Salmonella Dublin infections, antimicrobial drug resistance and clinical severity increased in parallel. In addition to the older age of patients and concurrent conditions often associated with Salmonella Dublin infections (7,11), we hypothesize that the multidrug resistance profile has led to the higher rates of treatment failure, prolonged hospitalizations, and higher mortality rates observed in our study.
Virulence factors may also contribute, particularly those factors located on resistance plasmids that are co-selected for when antimicrobial drugs are used in cattle. Salmonella Dublin has been described as having a serotype-specific virulence-associated plasmid that is associated with invasive infection and remains stable through multiple generations of nonselective bacterial passage (12). Additional analyses, with use of whole-genome sequencing, particularly methods like those developed by Pacific Biosciences (Menlo Park, CA, USA) to use long-sequence reads and facilitate plasmid analysis, would enable investigation into the respective contributions of virulence factors and resistance mechanisms.
The recently observed increase in human infections with Salmonella Dublin resistant to ceftriaxone and nalidixic acid probably resulted, in part, from the agricultural use of comparable antimicrobial drugs in animals. Over the past 15 years, ceftriaxone resistance among Salmonella Dublin isolates from FSIS-PR/HACCP (Pathogen Reduction/Hazard Analysis and Critical Control Point) samples from cattle increased from 0 to 86% (13). Davis et al. determined that among Salmonella Dublin isolates from cattle, resistance to the third-generation cephalosporin ceftazidime increased over a 5-year period; they suggested that antimicrobial resistance in Salmonella Dublin is probably driven by antimicrobial drug use in cattle without influence of antimicrobial drug use in humans (14). Berge et al. also observed increasing resistance to third-generation cephalosporins and fluoroquinolones in calves in California during 1998–2002 (15). These findings demonstrate that antimicrobial stewardship and judicious use programs are essential for maintaining the efficacy of drugs used in human and veterinary medicine.
Our data indicate that the incidence of Salmonella Dublin infections has increased while the incidence of other Salmonella infections has remained mostly stable (Figure 1). The peak in overall Salmonella infections that occurred in the mid-1980s was driven by a nationwide outbreak of Salmonella Typhimurium (16). The simultaneous spike in Salmonella Dublin resulted largely from consumption of raw milk (7,17), particularly from a large California dairy (18). The dairy producer promoted its raw milk as having health benefits (19), and many persons with compromised immune systems (e.g., young, elderly, or HIV-positive) became ill (20). As a result of a public health investigation (17), production and sales were halted, and FDA banned the interstate sale of raw milk in 1987 (21). A sharp decline in Salmonella Dublin infections soon followed.
Although additional data on food histories and the role of the environment will help elucidate the sources of human infections, the risk for Salmonella Dublin infection among humans is probably still caused, in part, by consumption of raw milk and beef. Raw dairy products have been linked to numerous Salmonella Dublin illnesses in the United States (17,22) and abroad (23,24). Incidence of Salmonella Dublin infections was 3 times higher in states that allow the sale of raw milk or permit cow shares than in states where raw milk sales are illegal. US surveillance data from PulseNet, the USDA Agricultural Marketing Service (25), and FDOSS also indicate that Salmonella Dublin has been isolated from ground beef and boneless beef products and has been associated with outbreak-associated illnesses from beef products. Salmonella Dublin has also been found in beef cattle and calves (26,27).
In our study, the higher proportion of Salmonella Dublin infections among men than women may be partially attributable to consumption patterns. Although the 2006–2007 FoodNet Population Survey found no differences by sex for consumption of raw milk or cheese items (28), numerous studies have found that men consume more beef and more undercooked beef than women (28,29). Occupational exposure to cattle may also contribute to the increased frequency of infection among men.
Most Salmonella Dublin infections continue to be reported from California (Figure 2), but illnesses have occurred nationwide. They are probably associated with an ongoing outbreak of Salmonella Dublin infections among US dairy and beef cattle. A 2014 dairy study conducted by the USDA National Animal Health Monitoring System found antibodies directed against Salmonella Dublin lipopolysaccharide O-antigens in 8% of bulk tank milk samples (30). Of operations in participating western states (California, Colorado, Idaho, Texas, and Washington), 52% were positive, compared with 2.8% of operations in eastern states (Kentucky, Michigan, Minnesota, Missouri, New York, Ohio, Pennsylvania, Vermont, Virginia, Wisconsin). In 2013, the Animal Health Diagnostic Center at Cornell University (Ithaca, NY, USA) issued an animal health advisory, warning cattle owners about an increase in MDR Salmonella Dublin infections among cattle in the northeastern United States, treatment difficulties associated with these infections, the potential for long-term environmental contamination, and the dangers (including death) that these infections pose to animals and humans (31).
Changes in the geographic distribution of Salmonella Dublin infections in cattle probably explain the similar geographic spread among humans. Historically, Salmonella Dublin in cattle was associated with the western United States and was not discovered in cattle east of the Rocky Mountains until 1968 (32). Salmonella Dublin continued to spread by transport of animals and their products and can now be found in cattle populations throughout the contiguous United States (26).
In Denmark, in response to the specific threat to human and animal health posed by Salmonella Dublin infections, in 2006, the Danish government passed legislation intended to eradicate this serotype. Their policy actions included heightened surveillance for cattle and abattoirs, voluntary interventions to reduce environmental contamination and disease spread within infected herds, economic sanctions for producers who do not control Salmonella Dublin in their herds, and closing of infected herds to live-animal trade (33,34). In the United States, precedent for the successful eradication of other host-adapted Salmonella serotypes in production animals has been set by use of vaccines and improved management practices. An example is the USDA National Poultry Improvement Plan, which has successfully eradicated Salmonella Gallinarum and Pullorum from domestic commercial poultry (35,36). Efforts are under way to decrease the burden of Salmonella Dublin among cattle. An oral modified-live Salmonella Dublin vaccine has been evaluated for use in calves; however, this vaccine has not been effective for reducing the incidence of disease, and research into finding an effective vaccine continues (37).
Interventions developed for the Denmark cattle and US poultry industries may not be completely applicable to the US cattle industry because of regulatory and production differences. For example, in Denmark, to control Salmonella Dublin infections, trade restrictions are applied to farms with affected herds, and in the United States, biosecurity procedures for poultry producers generally enable tighter environmental control than do those for cattle producers. However, judicious use of antimicrobial drugs in cattle, coupled with improved specific husbandry and management practices on the farm, could decrease antimicrobial-resistant Salmonella Dublin infection in cattle. In 2012, FDA prohibited certain extralabel uses of cephalosporins in chickens, turkeys, cattle, and swine (38). This new prohibition has the potential to slow the spread of cephalosporin resistance among food animals and is a valuable step toward protecting the effectiveness of current antimicrobial drugs. Nevertheless, other extralabel uses of cephalosporin drugs are still permitted.
Salmonella Dublin is a cattle-adapted Salmonella serotype that causes severe and antimicrobial drug–resistant infections in humans and cattle, and its incidence is on the rise. Reducing Salmonella Dublin carriage by cattle could benefit animal and human health. Unlike most other Salmonella infections in food animals, Salmonella Dublin can cause high mortality rates, particularly among calves, and heavy economic burdens for producers (39). It is well established that use of antimicrobial agents is a major driving force for the global surge in antimicrobial resistance. Food animal management practices, including veterinary use of antimicrobial drugs, may be contributing to the increasing resistance in Salmonella Dublin and to Salmonella Dublin–associated illness and death among humans (15). Therefore, careful evaluation of management practices and judicious use of antimicrobial drugs in cattle is critical for the control of antimicrobial drug–resistant Salmonella Dublin infections in cattle and humans. The 2016 FDA Veterinary Feed Directive aims to eliminate the use for food production purposes (i.e., growth promotion and feed efficiency) of antimicrobial drugs that are considered medically important in humans and seeks to bring all remaining therapeutic use of antimicrobial agents in feed and water under the oversight of licensed veterinarians (40). Agricultural and public health authorities will need to engage in ongoing, meaningful collaborations to reduce inappropriate antimicrobial use in food-producing animals to protect human and animal health.
Dr. Harvey completed this work as an Epidemic Intelligence Service Officer with the Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC. He is currently an epidemiologist with the CDC National Institute for Occupational Safety and Health in Morgantown, WV. His research interest is work-related lung disease in manufacturing industries.
References
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. DOIPubMedGoogle Scholar
- Su LH, Chiu CH. Salmonella: clinical importance and evolution of nomenclature. Chang Gung Med J. 2007;30:210–9.PubMedGoogle Scholar
- Solghan SM, Dumas NB, Root TP, Quinlan TM, Armstrong LR, Spina NL, et al. Multidrug-resistant nontyphoidal Salmonella in New York state’s foodborne diseases active surveillance network counties. Foodborne Pathog Dis. 2010;7:167–73. DOIPubMedGoogle Scholar
- Mølbak K. Human health consequences of antimicrobial drug-resistant Salmonella and other foodborne pathogens. Clin Infect Dis. 2005;41:1613–20. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): human isolates final report, 2013. Atlanta: The Centers; 2015.
- Wray C, Sojka WJ. Reviews of the progress of dairy science: bovine salmonellosis. J Dairy Res. 1977;44:383–425. DOIPubMedGoogle Scholar
- Taylor DN, Bied JM, Munro JS, Feldman RA. Salmonella dublin infections in the United States, 1979-1980. J Infect Dis. 1982;146:322–7. DOIPubMedGoogle Scholar
- Folster JP, Pecic G, Singh A, Duval B, Rickert R, Ayers S, et al. Characterization of extended-spectrum cephalosporin-resistant Salmonella enterica serovar Heidelberg isolated from food animals, retail meat, and humans in the United States 2009. Foodborne Pathog Dis. 2012;9:638–45. DOIPubMedGoogle Scholar
- Mungai EA, Behravesh CB, Gould LH. Increased outbreaks associated with nonpasteurized milk, United States, 2007-2012. Emerg Infect Dis. 2015;21:119–22. DOIPubMedGoogle Scholar
- Blaser MJ, Feldman RA. From the centers for disease control. Salmonella bacteremia: reports to the Centers for Disease Control, 1968-1979. J Infect Dis. 1981;143:743–6. DOIPubMedGoogle Scholar
- Fang FC, Fierer J. Human infection with Salmonella dublin. Medicine (Baltimore). 1991;70:198–207. DOIPubMedGoogle Scholar
- Olsen JE, Baggesen DL, Nielsen BB, Larsen HE. The prevalence of plasmids in Danish bovine and human isolates of Salmonella dublin. APMIS. 1990;98:735–40. DOIPubMedGoogle Scholar
- Food and Drug Administration. The 2012–2013 integrated NARMS report. Silver Spring (MD). Rev ADM. 2015.
- Davis MA, Hancock DD, Besser TE, Daniels JB, Baker KN, Call DR. Antimicrobial resistance in Salmonella enterica serovar Dublin isolates from beef and dairy sources. Vet Microbiol. 2007;119:221–30. DOIPubMedGoogle Scholar
- Berge AC, Thornburg E, Adaska JM, Moeller RB, Blanchard PC. Antimicrobial resistance in Salmonella enterica subspecies enterica serovar Dublin from dairy source calves in the central San Joaquin Valley, California (1998-2002). J Vet Diagn Invest. 2008;20:497–500. DOIPubMedGoogle Scholar
- Ryan CA, Nickels MK, Hargrett-Bean NT, Potter ME, Endo T, Mayer L, et al. Massive outbreak of antimicrobial-resistant salmonellosis traced to pasteurized milk. JAMA. 1987;258:3269–74. DOIPubMedGoogle Scholar
- Centers for Disease Control (CDC). Salmonella dublin and raw milk consumption—California. MMWR Morb Mortal Wkly Rep. 1984;33:196–8.PubMedGoogle Scholar
- Richwald GA, Greenland S, Johnson BJ, Friedland JM, Goldstein EJ, Plichta DT. Assessment of the excess risk of Salmonella dublin infection associated with the use of certified raw milk. Public Health Rep. 1988;103:489–93.PubMedGoogle Scholar
- Consumers Union of U.S., Inc. v. Alta-Dena Certified Dairy. 4 Cal App 4th 963: Court of Appeals of California, First District, Division Two; 1992.
- Stevens A. Safety of raw milk at issue in lawsuit filed against dairy. Los Angeles Times. December 5, 1988.
- Food and Drug Administration. Mandatory pasteurization for all milk and milk products in final package form intended for direct human consumption. 21CFR124061. Rockville (MD). Rev ADM. 1987.
- Werner SB, Humphrey GL, Kamei I. Association between raw milk and human Salmonella dublin infection. BMJ. 1979;2:238–41. DOIPubMedGoogle Scholar
- Ung A, Vignaud ML, Morand A, Donguy MP, Van Cauteren D, Lucas E, et al. Nationwide Salmonella Dublin outbreak associated with raw‐milk cheese consumption, France 2015–2016: results from a case-case study. International Symposium Salmonella and Salmonellosis; June 6–8, 2016; Saint-Malo, France.
- Vignaud ML, Marault M, Chaing E, Bachellerie P, Le Hello S. Michel Valérie, et al. MLVA for the subtyping of Salmonella enterica subsp. enterica serotype Dublin: report of France raw milk cheese outbreak in 2012. International Symposium Salmonella and Salmonellosis. June 6–8, 2016; Saint-Malo, France.
- Doerscher DR, Lutz TL, Whisenant SJ, Smith KR, Morris CA, Schroeder CM. Microbiological testing results of boneless and ground beef purchased for the national school lunch program, 2011 to 2014. J Food Prot. 2015;78:1656–63. DOIPubMedGoogle Scholar
- McDonough PL, Fogelman D, Shin SJ, Brunner MA, Lein DH. Salmonella enterica serotype Dublin infection: an emerging infectious disease for the northeastern United States. J Clin Microbiol. 1999;37:2418–27.PubMedGoogle Scholar
- McGuirk SM, Peek S. Salmonellosis in cattle: a review. American Association of Bovine Practitioners 36th Annual Conference. Sep 15–17, 2003; Columbus, Ohio [cited 2016 Nov 20]. http://www.vetmed.wisc.edu/dms/fapm/fapmtools/7health/Salmorev.pdf
- Shiferaw B, Verrill L, Booth H, Zansky SM, Norton DM, Crim S, et al. Sex-based differences in food consumption: Foodborne Diseases Active Surveillance Network (FoodNet) population survey, 2006–2007. Clin Infect Dis. 2012;54(Suppl 5):S453–7. DOIPubMedGoogle Scholar
- Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14:575–83. DOIPubMedGoogle Scholar
- Lombard JE, Thompson BS, Virkler PD, Wagner B, Kristensen C, Fossler C, et al. Salmonella Dublin antibodies in bulk-tank milk on U.S. dairy operations. Joint Annual Meeting of the American Society of Animal Science and the American Dairy Science Association. Jul 12–16, 2015; Orlando, Florida.
- Cornell University. Animal health advisory: multi-drug resistant Salmonella Dublin in cattle. Ithaca (NY): The University; 2013.
- Blackburn BO, Sutch K, Harrington R Jr. The changing distribution of Salmonella dublin in the United States. Proc Annu Meet U S Anim Health Assoc. 1980;84:445–51.PubMedGoogle Scholar
- Nielsen LR. National strategies of Salmonella control in cattle and beef in Denmark. 10th International Symposium on Veterinary Epidemiology and Economics. Nov 17–21, 2003; Vina del Mar, Chile; 2003.
- Nielsen LR, Nielsen SS. A structured approach to control of Salmonella Dublin in 10 Danish dairy herds based on risk scoring and test-and-manage procedures. Food Res Int. 2011;45:1158–65. DOIGoogle Scholar
- US Department of Agriculture, Animal and Plant Health Inspection Service. National poultry improvement plan [cited 2016 Feb 29]. http://www.poultryimprovement.org
- Hitchner SB. History of biological control of poultry diseases in the USA. Avian Dis. 2004;48:1–8. DOIPubMedGoogle Scholar
- Habing GG, Neuder LM, Raphael W, Piper-Youngs H, Kaneene JB. Efficacy of oral administration of a modified-live Salmonella Dublin vaccine in calves. J Am Vet Med Assoc. 2011;238:1184–90. DOIPubMedGoogle Scholar
- Food and Drug Administration. New animal drugs; cephalosporin drugs; extralabel animal drug use; order of prohibition. Federal Register; 2012. 77 FR 735. p. 735–45. Codified at 21 C.F.R. Sect 530.
- Peters AR. An estimation of the economic impact of an outbreak of Salmonella dublin in a calf rearing unit. Vet Rec. 1985;117:667–8. DOIPubMedGoogle Scholar
- Food and Drug Administration. Fact sheet: veterinary feed directive final rule and next steps [cited 2017 Jun 16]. https://www.fda.gov/animalveterinary/developmentapprovalprocess/ucm449019.htm
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers.
You must be a registered user on http://www.medscape.org. If you are not registered on http://www.medscape.org, please click on the “Register” link on the right hand side of the website.
Only one answer is correct for each question. Once you successfully answer all post-test questions, you will be able to view and/or print your certificate. For questions regarding this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please go to https://www.ama-assn.org. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate, and present it to your national medical association for review.
Article Title:
Epidemiology of Salmonella enterica Serotype Dublin Infections among Humans, United States, 1968–2013
CME Questions
1. You are advising a large health maintenance organization about anticipated needs regarding Salmonella serotype Dublin human bloodstream infections. On the basis of the analysis of US national surveillance data by Harvey and colleagues, which one of the following statements about incidence and demographics of Salmonella serotype Dublin human bloodstream infections is correct?
A. Incidence rate of Salmonella Dublin has overall been stable since 1968, except for a decrease and subsequent increase in incidence occurring throughout the 1980s
B. More than half of all Salmonella Dublin infections occurred in Oklahoma residents
C. 38% of Salmonella Dublin infections occurred in persons ≥65 years vs 11% of those with other Salmonella infections
D. History of international travel was significantly more common in Salmonella Dublin patients than in other Salmonella patients
2. According to the analysis of US national surveillance data by Harvey and colleagues, which one of the following statements about clinical severity of Salmonella serotype Dublin human bloodstream infections is correct?
A. Mortality rate decreased from 1996–2004 to 2005–2013
B. During 1996 to 2004, 58% of patients were hospitalized compared with 38% in 2005 to 2013
C. Reasons underlying the severity of Salmonella Dublin serotype infections are unknown
D. Salmonella Dublin has a serotype-specific virulence-associated plasmid that is associated with invasive infection and is stable through multiple generations of nonselective bacterial passage
3. On the basis of the analysis of US national surveillance data by Harvey and colleagues, which one of the following statements about antimicrobial resistance of Salmonella serotype Dublin human bloodstream infections is correct?
A. Resistance to 7 or more antimicrobial classes was present in 2.4% of isolates collected during 1996 to 2004 and in 8.8% of isolates collected during 2005 to 2013
B. Resistance to 3 or more antimicrobial classes was present in 21% of Salmonella Dublin isolates collected during 1996 to 2013 and in 12% of other Salmonella isolates
C. Less than 10% of Salmonella Dublin isolates were resistant to third-generation cephalosporins
D. Increases in human Salmonella Dublin infections with ceftriaxone and nalidixic acid resistance are likely caused in part by use of similar antimicrobials in animal agriculture
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
1Current affiliation: Centers for Disease Control and Prevention, Morgantown, WV, USA.
Related Links
Table of Contents – Volume 23, Number 9—September 2017
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Allison C. Brown, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop C16, Atlanta, GA 30329-4027
Top