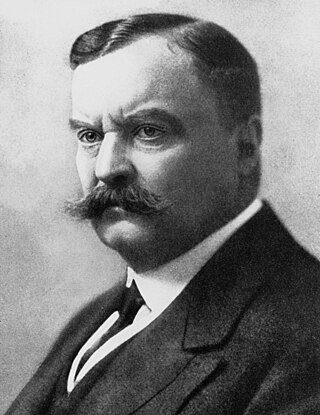Chagas disease, also known as American trypanosomiasis, is a tropical parasitic disease caused by Trypanosoma cruzi. It is spread mostly by insects in the subfamily Triatominae, known as "kissing bugs". The symptoms change over the course of the infection. In the early stage, symptoms are typically either not present or mild, and may include fever, swollen lymph nodes, headaches, or swelling at the site of the bite. After four to eight weeks, untreated individuals enter the chronic phase of disease, which in most cases does not result in further symptoms. Up to 45% of people with chronic infections develop heart disease 10–30 years after the initial illness, which can lead to heart failure. Digestive complications, including an enlarged esophagus or an enlarged colon, may also occur in up to 21% of people, and up to 10% of people may experience nerve damage.

African trypanosomiasis is an insect-borne parasitic infection of humans and other animals.
Trypanosomatida is a group of kinetoplastid unicellular organisms distinguished by having only a single flagellum. The name is derived from the Greek trypano (borer) and soma (body) because of the corkscrew-like motion of some trypanosomatid species. All members are exclusively parasitic, found primarily in insects. A few genera have life-cycles involving a secondary host, which may be a vertebrate, invertebrate or plant. These include several species that cause major diseases in humans. Some trypanosomatida are intracellular parasites, with the important exception of Trypanosoma brucei.

Tsetse are large, biting flies that inhabit much of tropical Africa. Tsetse flies include all the species in the genus Glossina, which are placed in their own family, Glossinidae. The tsetse is an obligate parasite, which lives by feeding on the blood of vertebrate animals. Tsetse has been extensively studied because of their role in transmitting disease. They have pronounced economic and public health impacts in sub-Saharan Africa as the biological vectors of trypanosomes, causing human and animal trypanosomiasis.

Trypanosomiasis or trypanosomosis is the name of several diseases in vertebrates caused by parasitic protozoan trypanosomes of the genus Trypanosoma. In humans this includes African trypanosomiasis and Chagas disease. A number of other diseases occur in other animals.

Carlos Justiniano Ribeiro Chagas, or Carlos Chagas, was a Brazilian sanitary physician, scientist, and microbiologist who worked as a clinician and researcher. Most well known for the discovery of an eponymous protozoal infection called Chagas disease, also called American trypanosomiasis, he also discovered the causative fungi of the pneumocystis pneumonia. He described the two pathogens in 1909, while he was working at the Oswaldo Cruz Institute in Rio de Janeiro, and named the former Trypanosoma cruzi to honour his friend Oswaldo Cruz.
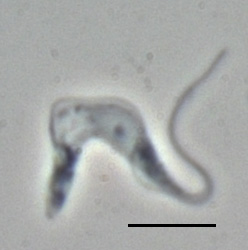
Trypanosoma brucei is a species of parasitic kinetoplastid belonging to the genus Trypanosoma that is present in sub-Saharan Africa. Unlike other protozoan parasites that normally infect blood and tissue cells, it is exclusively extracellular and inhabits the blood plasma and body fluids. It causes deadly vector-borne diseases: African trypanosomiasis or sleeping sickness in humans, and animal trypanosomiasis or nagana in cattle and horses. It is a species complex grouped into three subspecies: T. b. brucei, T. b. gambiense and T. b. rhodesiense. The first is a parasite of non-human mammals and causes nagana, while the latter two are zoonotic infecting both humans and animals and cause African trypanosomiasis.

Trypanosoma evansi is a parasitic species of excavate trypanosome in the genus Trypanosoma that is one cause of surra in animals. Discovered by Griffith Evans in 1880 at Dera Ismail Khan, it is the first known trypanosome that causes infection. It is a common parasite in India and Iran and causes acute disease in camels and horses, and chronic disease in cattle and buffalo. In Pakistan, it has been found to be the most prevalent trypanosome species in donkeys. It is now established to infect other mammals, including humans.
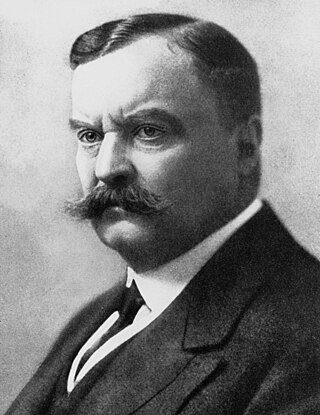
Major-General Sir David Bruce, was a Scottish pathologist and microbiologist who made some of the key contributions in tropical medicine. In 1887, he discovered a bacterium, now called Brucella, that caused what was known as Malta fever. In 1894, he discovered a protozoan parasite, named Trypanosoma brucei, as the causative pathogen of nagana.
Trypanosoma suis is a species of excavate trypanosome in the genus Trypanosoma that causes one form of the surra disease in animals. It infects pigs. It does not infect humans.

Animal trypanosomiasis, also known as nagana and nagana pest, or sleeping sickness, is a disease of vertebrates. The disease is caused by trypanosomes of several species in the genus Trypanosoma such as T. brucei. T. vivax causes nagana mainly in West Africa, although it has spread to South America. The trypanosomes infect the blood of the vertebrate host, causing fever, weakness, and lethargy, which lead to weight loss and anemia; in some animals the disease is fatal unless treated. The trypanosomes are transmitted by tsetse flies.

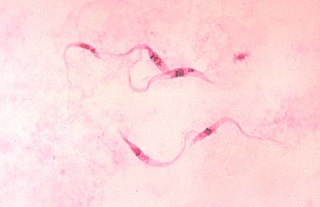
Trypanosoma cruzi is a species of parasitic euglenoids. Among the protozoa, the trypanosomes characteristically bore tissue in another organism and feed on blood (primarily) and also lymph. This behaviour causes disease or the likelihood of disease that varies with the organism: Chagas disease in humans, dourine and surra in horses, and a brucellosis-like disease in cattle. Parasites need a host body and the haematophagous insect triatomine is the major vector in accord with a mechanism of infection. The triatomine likes the nests of vertebrate animals for shelter, where it bites and sucks blood for food. Individual triatomines infected with protozoa from other contact with animals transmit trypanosomes when the triatomine deposits its faeces on the host's skin surface and then bites. Penetration of the infected faeces is further facilitated by the scratching of the bite area by the human or animal host.
Paratransgenesis is a technique that attempts to eliminate a pathogen from vector populations through transgenesis of a symbiont of the vector. The goal of this technique is to control vector-borne diseases. The first step is to identify proteins that prevent the vector species from transmitting the pathogen. The genes coding for these proteins are then introduced into the symbiont, so that they can be expressed in the vector. The final step in the strategy is to introduce these transgenic symbionts into vector populations in the wild. One use of this technique is to prevent mortality for humans from insect-borne diseases. Preventive methods and current controls against vector-borne diseases depend on insecticides, even though some mosquito breeds may be resistant to them. There are other ways to fully eliminate them. “Paratransgenesis focuses on utilizing genetically modified insect symbionts to express molecules within the vector that are deleterious to pathogens they transmit.” The acidic bacteria Asaia symbionts are beneficial in the normal development of mosquito larvae; however, it is unknown what Asais symbionts do to adult mosquitoes.

Nifurtimox, sold under the brand name Lampit, is a medication used to treat Chagas disease and sleeping sickness. For sleeping sickness it is used together with eflornithine in nifurtimox-eflornithine combination treatment. In Chagas disease it is a second-line option to benznidazole. It is given by mouth.
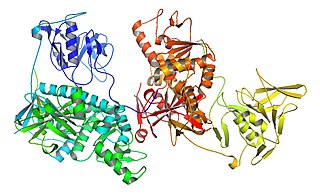
In enzymology, a trypanothione synthase (EC 6.3.1.9) is an enzyme that catalyzes the chemical reaction

Trypanosoma congolense is a species of trypanosomes and is the major pathogen responsible for the disease nagana in cattle and other animals including sheep, pigs, goats, horses and camels, dogs, as well as laboratory mice. It is the most common cause of nagana in east Africa, but is also a major cause of nagana in west Africa. This parasite is spread by tsetse flies. In its mammalian host, Trypanosoma congolense only lives in blood vessels, and causes in particular anaemia.
Trypanosoma rangeli is a species of hemoflagellate excavate parasites of the genus Trypanosoma. Although infecting a variety of mammalian species in a wide geographical area in Central and South America, this parasite is considered non-pathogenic to these hosts. T. rangeli is transmitted by bite of infected triatomine bugs of the Reduviidae family, commonly known as barbeiro, winchuka(vinchuca), chinche, pito ou chupão.
Wendy Gibson is Professor of Protozoology at University of Bristol, specialising in trypanosomes and molecular parasitology.
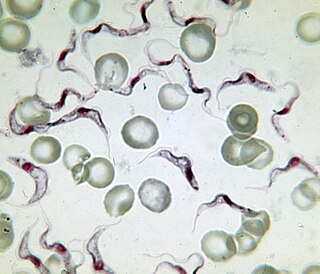
Variant surface glycoprotein (VSG) is a ~60kDa protein which densely packs the cell surface of protozoan parasites belonging to the genus Trypanosoma. This genus is notable for their cell surface proteins. They were first isolated from Trypanosoma brucei in 1975 by George Cross. VSG allows the trypanosomatid parasites to evade the mammalian host's immune system by extensive antigenic variation. They form a 12–15 nm surface coat. VSG dimers make up ~90% of all cell surface protein and ~10% of total cell protein. For this reason, these proteins are highly immunogenic and an immune response raised against a specific VSG coat will rapidly kill trypanosomes expressing this variant. However, with each cell division there is a possibility that the progeny will switch expression to change the VSG that is being expressed. VSG has no prescribed biochemical activity.
The Sleeping Sickness Commission was a medical project established by the British Royal Society to investigate the outbreak of African sleeping sickness or African trypanosomiasis in Africa at the turn of the 20th century. The outbreak of the disease started in 1900 in Uganda, which was at the time a protectorate of the British Empire. The initial team in 1902 consisted of Aldo Castellani and George Carmichael Low, both from the London School of Hygiene and Tropical Medicine, and Cuthbert Christy, a medical officer on duty in Bombay, India. From 1903, David Bruce of the Royal Army Medical Corps and David Nunes Nabarro of the University College Hospital took over the leadership. The commission established that species of blood protozoan called Trypanosoma brucei, named after Bruce, was the causative parasite of sleeping sickness.