
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.

Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond as a form of carbon is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.

Silicon carbide (SiC), also known as carborundum, is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.

Moissanite is naturally occurring silicon carbide and its various crystalline polymorphs. It has the chemical formula SiC and is a rare mineral, discovered by the French chemist Henri Moissan in 1893. Silicon carbide or moissanite is useful for commercial and industrial applications due to its hardness, optical properties and thermal conductivity.

Carbon is capable of forming many allotropes due to its valency (tetravalent). Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.

A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.
Gemesis Inc. was a privately held company located in New York City. The company grew synthetic diamonds using proprietary technology.

Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. It is a crystal that is transparent to opaque and which is generally isotropic. Diamond is the hardest naturally occurring material known. Yet, due to important structural brittleness, bulk diamond's toughness is only fair to good. The precise tensile strength of bulk diamond is little known; however, compressive strength up to 60 GPa has been observed, and it could be as high as 90–100 GPa in the form of micro/nanometer-sized wires or needles, with a corresponding maximum tensile elastic strain in excess of 9%. The anisotropy of diamond hardness is carefully considered during diamond cutting. Diamond has a high refractive index (2.417) and moderate dispersion (0.044) properties that give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defects present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal structure, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators and extremely efficient thermal conductors. Unlike many other minerals, the specific gravity of diamond crystals (3.52) has rather small variation from diamond to diamond.
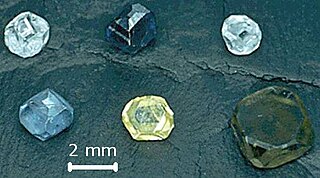
Imperfections in the crystal lattice of diamond are common. Such defects may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth. The defects affect the material properties of diamond and determine to which type a diamond is assigned; the most dramatic effects are on the diamond color and electrical conductivity, as explained by the electronic band structure.

Carbon nanofibers (CNFs), vapor grown carbon fibers (VGCFs), or vapor grown carbon nanofibers (VGCNFs) are cylindrical nanostructures with graphene layers arranged as stacked cones, cups or plates. Carbon nanofibers with graphene layers wrapped into perfect cylinders are called carbon nanotubes.
A multi-anvil press, or anvil press is a type of device related to a machine press that is used to create extraordinarily high pressures within a small volume.
Memorial diamonds are lab-grown diamonds that are promoted as being produced from carbon extracted from human or pet animal hair or cremated remains, cremation diamonds, that are provided to the producer by the customer.

BARS a high-pressure high-temperature apparatus usually used for growing or processing minerals, especially diamond and boron nitride. Typical pressures and temperatures achievable with BARS are 10 GPa (99,000 atm) and 2,500 °C.

Covalent superconductors are superconducting materials where the atoms are linked by covalent bonds. The first such material was boron-doped synthetic diamond grown by the high-pressure high-temperature (HPHT) method. The discovery had no practical importance, but surprised most scientists as superconductivity had not been observed in covalent semiconductors, including diamond and silicon.
WD Lab Grown Diamonds was a manufacturer of synthetic chemical vapor deposition (CVD) diamonds, headquartered in the Washington, D.C. area. Founded in 2008, WD produced lab-grown diamonds for distribution under the brands WD Lab Grown Diamonds and Latitude, in addition to creating diamonds for high-tech Advanced Materials applications. The company formally pivoted and relaunched as WD Advanced Materials, LLC in November 2023.
A rapidly increasing list of graphene production techniques have been developed to enable graphene's use in commercial applications.

Techniques have been developed to produce carbon nanotubes (CNTs) in sizable quantities, including arc discharge, laser ablation, high-pressure carbon monoxide disproportionation, and chemical vapor deposition (CVD). Most of these processes take place in a vacuum or with process gases. CVD growth of CNTs can occur in a vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth are making CNTs more commercially viable.
In materials science, vertically aligned carbon nanotube arrays (VANTAs) are a unique microstructure consisting of carbon nanotubes oriented with their longitudinal axis perpendicular to a substrate surface. These VANTAs effectively preserve and often accentuate the unique anisotropic properties of individual carbon nanotubes and possess a morphology that may be precisely controlled. VANTAs are consequently widely useful in a range of current and potential device applications.

























