
Bromine is a chemical element with the symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.
Silver sulfide is an inorganic compound with the formula Ag
2S. A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in most solvents, but is degraded by strong acids. Silver sulfide is a network solid made up of silver and sulfur where the bonds have low ionic character.

Di-tert-butyl ether is a tertiary ether, primarily of theoretical interest as the simplest member of the class of di-tertiary ethers.
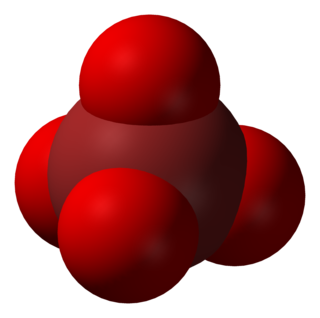
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.
Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by adding four hydrogens. In 2013, its preferred IUPAC name was established as oxane. The compound is a colourless volatile liquid. Derivatives of tetrahydropyran are, however, more common. 2-Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and 3,4-dihydropyran are commonly used as protecting groups in organic synthesis. Furthermore, a tetrahydropyran ring system, i.e., five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.
Aluminium iodide is a chemical compound containing aluminium and iodine. Invariably, the name refers to a compound of the composition AlI
3, formed by the reaction of aluminium and iodine or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. Like the related chloride and bromide, AlI
3 is a strong Lewis acid and will absorb water from the atmosphere. It is employed as a reagent for the scission of certain kinds of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
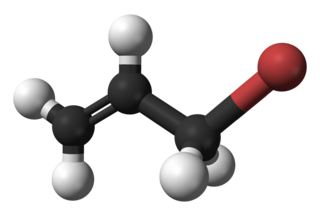
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and persistent smell, however, commercial samples are yellow or brown. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.
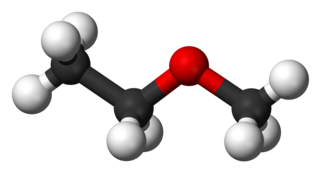
Methoxyethane, also known as ethyl methyl ether, is a colorless gaseous ether. Unlike the related dimethyl ether and diethyl ether, which are widely used and studied, this mixed alkyl ether has no current applications. Its utility as an anesthetic and solvent have been investigated.

A dough conditioner, flour treatment agent, improving agent or bread improver is any ingredient or chemical added to bread dough to strengthen its texture or otherwise improve it in some way. Dough conditioners may include enzymes, yeast nutrients, mineral salts, oxidants and reductants, bleaching agents and emulsifiers. They are food additives combined with flour to improve baking functionality. Flour treatment agents are used to increase the speed of dough rising and to improve the strength and workability of the dough. While they are an important component of modern factory baking, some small-scale bakers reject them in favour of longer fermentation periods that produce greater depth of flavour.
3-Aminobenzoic acid (also known as meta-aminobenzoic acid or MABA) is an organic compound with the molecular formula H2NC6H4CO2H. MABA is a white solid, although commercial samples are often colored. It is only slightly soluble in water. It is soluble in acetone, boiling water, hot alcohol, hot chloroform and ether. It consists of a benzene ring substituted with an amino group and a carboxylic acid.
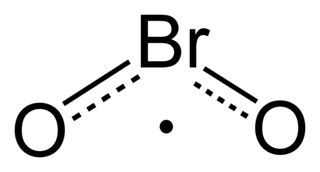
Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow to yellow-orange crystals. It was first isolated by R. Schwarz and M. Schmeißer in 1937 and is hypothesized to be important in the atmospheric reaction of bromine with ozone. It is similar to chlorine dioxide, the dioxide of its halogen neighbor one period higher on the periodic table.
Calcium bromate, Ca(BrO3)2, is a calcium salt of bromic acid. It is most commonly encountered as the monohydrate, Ca(BrO3)2•H2O.
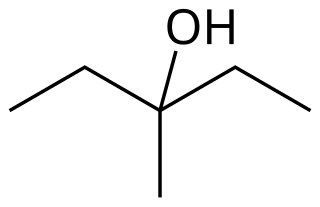
3-Methyl-3-pentanol is an organic chemical compound and a tertiary hexanol. It is used in the synthesis of the tranquilizer emylcamate, and has similar sedative and anticonvulsant actions itself.
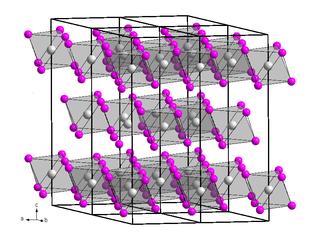
Chromium(III) bromide is an inorganic compound with the chemical formula CrBr3. It is a dark colored solid that appears green in transmitted light but red with reflected light. It is used as a precursor to catalysts for the oligomerization of ethylene.
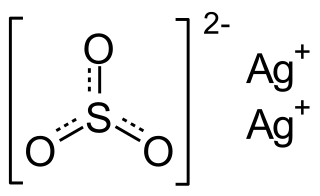
Silver sulfite is the chemical compound with the formula Ag2SO3. This unstable silver compound when heated and/or in light it decomposes to silver dithionate and silver sulfate.
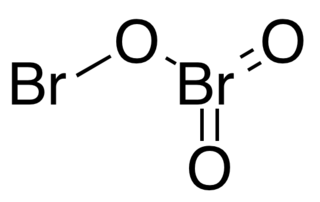
Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula Br2O3. It is an orange solid that is stable below −40 °C. It has the structure Br−O−BrO2 (bromine bromate). The Br−O−Br bond is bent, with a bond angle of 111.2°, and the Br−O−BrO2 bond length is 1.85Å.
Barium bromate is a chemical compound composed of the barium ion and the bromate ion, with the chemical formula of Ba(BrO3)2.

















