Related Research Articles

Staphylococcus aureus is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

Methicillin-resistant Staphylococcus aureus (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019.

Fusidic acid, sold under the brand names Fucidin among others, is an antibiotic that is often used topically in creams or ointments and eyedrops but may also be given systemically as tablets or injections.
As of October 2008, the global problem of advancing antimicrobial resistance has led to a renewed interest in its use.

Vancomycin-resistant Staphylococcus aureus (VRSA) are strains of Staphylococcus aureus that have acquired resistance to the glycopeptide antibiotic vancomycin. Bacteria can acquire resistant genes either by random mutation or through the transfer of DNA from one bacterium to another. Resistance genes interfere with the normal antibiotic function and allow a bacteria to grow in the presence of the antibiotic. Resistance in VRSA is conferred by the plasmid-mediated vanA gene and operon. Although VRSA infections are uncommon, VRSA is often resistant to other types of antibiotics and a potential threat to public health because treatment options are limited. VRSA is resistant to many of the standard drugs used to treat S. aureus infections. Furthermore, resistance can be transferred from one bacterium to another.

Staphylococcus haemolyticus is a member of the coagulase-negative staphylococci (CoNS). It is part of the skin flora of humans, and its largest populations are usually found at the axillae, perineum, and inguinal areas. S. haemolyticus also colonizes primates and domestic animals. It is a well-known opportunistic pathogen, and is the second-most frequently isolated CoNS. Infections can be localized or systemic, and are often associated with the insertion of medical devices. The highly antibiotic-resistant phenotype and ability to form biofilms make S. haemolyticus a difficult pathogen to treat. Its most closely related species is Staphylococcus borealis.

Pristinamycin (INN), also spelled pristinamycine, is an antibiotic used primarily in the treatment of staphylococcal infections, and to a lesser extent streptococcal infections. It is a streptogramin group antibiotic, similar to virginiamycin, derived from the bacterium Streptomyces pristinaespiralis. It is marketed in Europe by Sanofi-Aventis under the trade name Pyostacine.

Panton–Valentine leukocidin (PVL) is a cytotoxin—one of the β-pore-forming toxins. The presence of PVL is associated with increased virulence of certain strains (isolates) of Staphylococcus aureus. It is present in the majority of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) isolates studied and is the cause of necrotic lesions involving the skin or mucosa, including necrotic hemorrhagic pneumonia. PVL creates pores in the membranes of infected cells. PVL is produced from the genetic material of a bacteriophage that infects Staphylococcus aureus, making it more virulent.
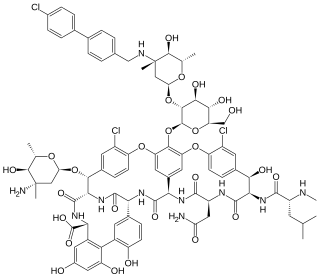
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.
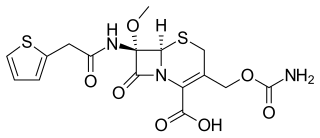
Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.
Lysostaphin is a Staphylococcus simulans metalloendopeptidase. It can function as a bacteriocin (antimicrobial) against Staphylococcus aureus.
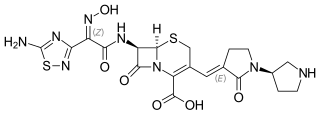
Ceftobiprole (Zevtera/Mabelio) is a fifth-generation cephalosporin for the treatment of hospital-acquired pneumonia and community-acquired pneumonia. It is marketed by Basilea Pharmaceutica in the United Kingdom, Germany, Switzerland and Austria under the trade name Zevtera, in France and Italy under the trade name Mabelio. Like other cephalosporins, ceftobiprole exerts its antibacterial activity by binding to important penicillin-binding proteins and inhibiting their transpeptidase activity which is essential for the synthesis of bacterial cell walls. Ceftobiprole has high affinity for penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus strains and retains its activity against strains that express divergent mecA gene homologues. Ceftobiprole also binds to penicillin-binding protein 2b in Streptococcus pneumoniae (penicillin-intermediate), to penicillin-binding protein 2x in Streptococcus pneumoniae (penicillin-resistant), and to penicillin-binding protein 5 in Enterococcus faecalis.
Phenol-soluble modulins (PSMs) are a family of small proteins, that carry out a variety of functions, including acting as toxins, assisting in biofilm formation, and colony spreading. PSMs are produced by Staphylococcus bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus epidermidis. Many PSMs are encoded within the core genome and can play an important virulence factor. PSMs were first discovered in S. epidermidis by Seymour Klebanoff and via hot-phenol extraction and were described as a pro-inflammatory complex of three peptides. Since their initial discovery, numerous roles of PSMs have been identified. However, due in part to the small size of many PSMs, they have largely gone unnoticed until recent years.
mecA is a gene found in bacterial cells which allows them to be resistant to antibiotics such as methicillin, penicillin and other penicillin-like antibiotics.

Staphylococcus is a genus of Gram-positive bacteria in the family Staphylococcaceae from the order Bacillales. Under the microscope, they appear spherical (cocci), and form in grape-like clusters. Staphylococcus species are facultative anaerobic organisms.
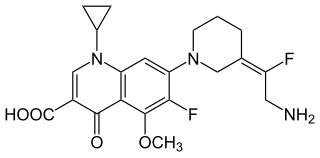
JNJ-Q2 is a broad-spectrum fluoroquinolone antibacterial drug being developed for the treatment of acute bacterial skin and skin-structure infections and community-acquired pneumonia. Specifically, JNJ-Q2 is being actively studied for treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections.
Staphylococcus schleiferi is a Gram-positive, cocci-shaped bacterium of the family Staphylococcaceae. It is facultatively anaerobic, coagulase-variable, and can be readily cultured on blood agar where the bacterium tends to form opaque, non-pigmented colonies and beta (β) hemolysis. There exists two subspecies under the species S. schleiferi: Staphylococcus schleiferi subsp. schleiferi and Staphylococcus schleiferi subsp. coagulans.
Staphylococcus pseudintermedius is a gram positive coccus bacteria of the genus Staphylococcus found worldwide. It is primarily a pathogen for domestic animals, but has been known to affect humans as well. S. pseudintermedius is an opportunistic pathogen that secretes immune modulating virulence factors, has many adhesion factors, and the potential to create biofilms, all of which help to determine the pathogenicity of the bacterium. Diagnoses of Staphylococcus pseudintermedius have traditionally been made using cytology, plating, and biochemical tests. More recently, molecular technologies like MALDI-TOF, DNA hybridization and PCR have become preferred over biochemical tests for their more rapid and accurate identifications. This includes the identification and diagnosis of antibiotic resistant strains.
The arginine catabolic mobile element (ACME) is a mobile genetic element of Staphylococcus bacterial species. This genetic element provides for several immune modulating functions, including resistance to polyamines which serve as a non-specific immune response both on intact skin and following the inflammatory response in wound healing. Diverse ACME are present in several species of Staphylococcus, including Staphylococcus epidermidis.
Accessory gene regulator (agr) is a complex 5 gene locus that is a global regulator of virulence in Staphylococcus aureus. It encodes a two-component transcriptional quorum-sensing (QS) system activated by an autoinducing, thiolactone-containing cyclic peptide (AIP).
Kerry L. LaPlante is an American pharmacist, academic and researcher. She is a Professor of Pharmacy and the Chair of the Department of Pharmacy Practice at the University of Rhode Island, an Adjunct Professor of Medicine at Brown University, an Infectious Diseases Pharmacotherapy Specialist, and the Director of the Rhode Island Infectious Diseases Fellowship and Research Programs at the Veterans Affairs Medical Center in Providence, Rhode Island.
References
- ↑ Hanssen AM, Ericson Sollid JU (February 2006). "SCCmec in staphylococci: genes on the move". FEMS Immunology and Medical Microbiology. 46 (1): 8–20. doi: 10.1111/j.1574-695X.2005.00009.x . PMID 16420592. S2CID 37999833.
- ↑ Katayama Y, Ito T, Hiramatsu K (June 2000). "A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus". Antimicrobial Agents and Chemotherapy. 44 (6): 1549–1555. doi:10.1128/AAC.44.6.1549-1555.2000. PMC 89911 . PMID 10817707.
- ↑ Ito T, Hiramatsu K (December 1998). "Acquisition of methicillin resistance and progression of multiantibiotic resistance in methicillin-resistant Staphylococcus aureus". Yonsei Medical Journal. 39 (6): 526–533. doi: 10.3349/ymj.1998.39.6.526 . PMID 10097679.
- ↑ Hanssen AM, Ericson Sollid JU (February 2006). "SCCmec in staphylococci: genes on the move". FEMS Immunology and Medical Microbiology. 46 (1): 8–20. doi: 10.1111/j.1574-695X.2005.00009.x . PMID 16420592.
- ↑ Uehara Y (January 2022). "Current Status of Staphylococcal Cassette Chromosome mec (SCCmec)". Antibiotics. 11 (1): 86. doi: 10.3390/antibiotics11010086 . PMC 8772726 . PMID 35052963.
- ↑ Hiramatsu K, Cui L, Kuroda M, Ito T (October 2001). "The emergence and evolution of methicillin-resistant Staphylococcus aureus". Trends in Microbiology. 9 (10): 486–493. doi:10.1016/s0966-842x(01)02175-8. PMID 11597450.
- 1 2 Katayama Y, Ito T, Hiramatsu K (July 2001). "Genetic organization of the chromosome region surrounding mecA in clinical staphylococcal strains: role of IS431-mediated mecI deletion in expression of resistance in mecA-carrying, low-level methicillin-resistant Staphylococcus haemolyticus". Antimicrobial Agents and Chemotherapy. 45 (7): 1955–1963. doi:10.1128/AAC.45.7.1955-1963.2001. PMC 90585 . PMID 11408208.
- ↑ Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, et al. (August 2011). "Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus". Antimicrobial Agents and Chemotherapy. 55 (8): 3765–3773. doi:10.1128/AAC.00187-11. PMC 3147645 . PMID 21636525.
- ↑ "Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements". Antimicrobial Agents and Chemotherapy. 53 (12): 4961–4967. December 2009. doi:10.1128/aac.00579-09. PMC 2786320 . PMID 19721075.
- ↑ "Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements". Antimicrobial Agents and Chemotherapy. 53 (12): 4961–4967. December 2009. doi:10.1128/AAC.00579-09. PMC 2786320 . PMID 19721075.
- ↑ Liu J, Chen D, Peters BM, Li L, Li B, Xu Z, Shirliff ME (December 2016). "Staphylococcal chromosomal cassettes mec (SCCmec): A mobile genetic element in methicillin-resistant Staphylococcus aureus". Microbial Pathogenesis. 101: 56–67. doi:10.1016/j.micpath.2016.10.028. PMID 27836760.
- ↑ Lakhundi S, Zhang K (October 2018). "Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology". Clinical Microbiology Reviews. 31 (4). doi:10.1128/CMR.00020-18. PMC 6148192 . PMID 30209034.
- ↑ Tsubakishita S, Kuwahara-Arai K, Sasaki T, Hiramatsu K (October 2010). "Origin and molecular evolution of the determinant of methicillin resistance in staphylococci". Antimicrobial Agents and Chemotherapy. 54 (10): 4352–4359. doi:10.1128/AAC.00356-10. PMC 2944575 . PMID 20679504.
- ↑ Tulinski P, Fluit AC, Wagenaar JA, Mevius D, van de Vijver L, Duim B (January 2012). "Methicillin-resistant coagulase-negative staphylococci on pig farms as a reservoir of heterogeneous staphylococcal cassette chromosome mec elements". Applied and Environmental Microbiology. 78 (2): 299–304. Bibcode:2012ApEnM..78..299T. doi:10.1128/AEM.05594-11. PMC 3255757 . PMID 22081567.