
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.
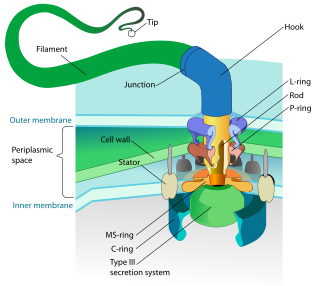
A flagellum is a hair-like appendage that protrudes from certain plant and animal sperm cells, from fungal spores (zoospores), and from a wide range of microorganisms to provide motility. Many protists with flagella are known as flagellates.

The cilium is a short hair-like membrane protrusion from many types of eukaryotic cell. The cilium has the shape of a slender threadlike projection that extends from the surface of the much larger cell body. Eukaryotic flagella found on sperm cells and many protozoans have a similar structure to motile cilia that enables swimming through liquids; they are longer than cilia and have a different undulating motion.
The evolution of flagella is of great interest to biologists because the three known varieties of flagella – each represent a sophisticated cellular structure that requires the interaction of many different systems.
The microtubule-organizing center (MTOC) is a structure found in eukaryotic cells from which microtubules emerge. MTOCs have two main functions: the organization of eukaryotic flagella and cilia and the organization of the mitotic and meiotic spindle apparatus, which separate the chromosomes during cell division. The MTOC is a major site of microtubule nucleation and can be visualized in cells by immunohistochemical detection of γ-tubulin. The morphological characteristics of MTOCs vary between the different phyla and kingdoms. In animals, the two most important types of MTOCs are 1) the basal bodies associated with cilia and flagella and 2) the centrosome associated with spindle formation.

Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport.

In molecular biology, an axoneme, also called an axial filament, is the microtubule-based cytoskeletal structure that forms the core of a cilium or flagellum. Cilia and flagella are found on many cells, organisms, and microorganisms, to provide motility. The axoneme serves as the "skeleton" of these organelles, both giving support to the structure and, in some cases, the ability to bend. Though distinctions of function and length may be made between cilia and flagella, the internal structure of the axoneme is common to both.
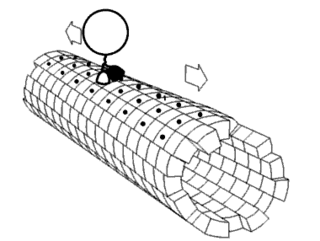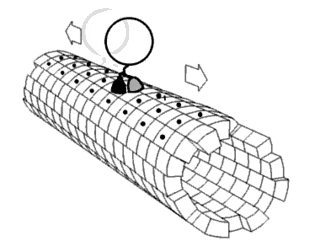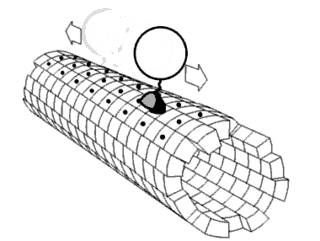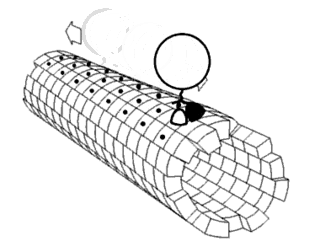
Motor proteins are a class of molecular motors that can move along the cytoskeleton of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.

Intraflagellar transport (IFT) is a bidirectional motility along axoneme microtubules that is essential for the formation (ciliogenesis) and maintenance of most eukaryotic cilia and flagella. It is thought to be required to build all cilia that assemble within a membrane projection from the cell surface. Plasmodium falciparum cilia and the sperm flagella of Drosophila are examples of cilia that assemble in the cytoplasm and do not require IFT. The process of IFT involves movement of large protein complexes called IFT particles or trains from the cell body to the ciliary tip and followed by their return to the cell body. The outward or anterograde movement is powered by kinesin-2 while the inward or retrograde movement is powered by cytoplasmic dynein 2/1b. The IFT particles are composed of about 20 proteins organized in two subcomplexes called complex A and B.
An undulipodium or undulopodium, or a 9+2 organelle is a motile filamentous extracellular projection of eukaryotic cells. It is basically synonymous to flagella and cilia which are differing terms for similar molecular structures used on different types of cells, and usually correspond to different waveforms.

Sperm motility describes the ability of sperm to move properly through the female reproductive tract or through water to reach the egg. Sperm motility can also be thought of as the quality, which is a factor in successful conception; sperm that do not "swim" properly will not reach the egg in order to fertilize it. Sperm motility in mammals also facilitates the passage of the sperm through the cumulus oophorus and the zona pellucida, which surround the mammalian oocyte.

Kinesin-like protein KIF3A is a protein that in humans is encoded by the KIF3A gene.

Kinesin-like protein KIF3B is a protein that in humans is encoded by the KIF3B gene. KIF3B is an N-type protein that complexes with two other kinesin proteins to form two-headed anterograde motors. First, KIF3B forms a heterodimer with KIF3A ; (KIF3A/3B), that is membrane-bound and has ATPase activity. Then KIFAP3 binds to the tail domain to form a heterotrimeric motor. This motor has a plus end-directed microtubule sliding activity that exhibits a velocity of ~0.3 μm/s a. There are 14 kinesin protein families in the kinesin superfamily and KIF3B is part of the Kinesin-2 family, of kinesins that can all form heterotrimeric complexes. Expression of the three motor subunits is ubiquitous. The KIG3A/3B/KAP3 motors can transport 90 to 160 nm in diameter organelles.
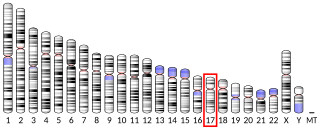
Dynein heavy chain 9, axonemal is a protein that in humans is encoded by the DNAH9 gene.

Dynein axonemal intermediate chain 1 is a protein that in humans is encoded by the DNAI1 gene.

Radial spoke head protein 4 homolog A, also known as radial spoke head-like protein 3, is a protein that in humans is encoded by the RSPH4A gene.

Ciliogenesis is defined as the building of the cell's antenna or extracellular fluid mediation mechanism. It includes the assembly and disassembly of the cilia during the cell cycle. Cilia are important appendages of cells and are involved in numerous activities such as cell signaling, processing developmental signals, and directing the flow of fluids such as mucus over and around cells. Due to the importance of these cell processes, defects in ciliogenesis can lead to numerous human diseases related to non-functioning cilia known as ciliopathies.

Ian Read Gibbons, was a biophysicist and cell biologist. He discovered and named dynein, and demonstrated energy source as ATP is sufficient for dynein to walk on microtubules. In 2017, he and Ronald Vale received the Shaw Prize for their research on microtubule motor proteins.
Gaia Pigino is the Associate Head of the Structural Biology Research Center and Leader of the "Pigino Group" at the Human Technopole in Milan, Italy.















