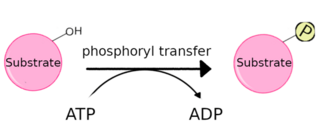
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.
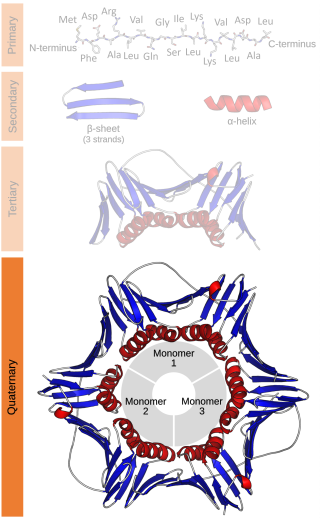
Protein quaternary structure is the fourth classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains. Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors.
In chemistry, dimerization is the process of joining two identical or similar molecular entities by bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is unknown or highly unstable.
DNA primase is an enzyme involved in the replication of DNA and is a type of RNA polymerase. Primase catalyzes the synthesis of a short RNA segment called a primer complementary to a ssDNA template. After this elongation, the RNA piece is removed by a 5' to 3' exonuclease and refilled with DNA.
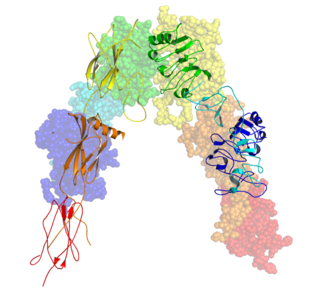
The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis; a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene INSR, from which alternate splicing during transcription results in either IR-A or IR-B isoforms. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon combination are ultimately capable of homo or hetero-dimerisation to produce the ≈320 kDa disulfide-linked transmembrane insulin receptor.

The enzyme alkaline phosphatase is a phosphatase with the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function, but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of E. coli bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development within the skeleton. Due to its widespread prevalence in these areas, its concentration in the bloodstream is used by diagnosticians as a biomarker in helping determine diagnoses such as hepatitis or osteomalacia.
Thioredoxin reductases are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond.

A basic helix–loop–helix (bHLH) is a protein structural motif that characterizes one of the largest families of dimerizing transcription factors. The word "basic" does not refer to complexity but to the chemistry of the motif because transcription factors in general contain basic amino acid residues in order to facilitate DNA binding.
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits, and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits, or two heterodimer subunits.
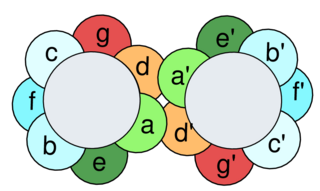
A leucine zipper is a common three-dimensional structural motif in proteins. They were first described by Landschulz and collaborators in 1988 when they found that an enhancer binding protein had a very characteristic 30-amino acid segment and the display of these amino acid sequences on an idealized alpha helix revealed a periodic repetition of leucine residues at every seventh position over a distance covering eight helical turns. The polypeptide segments containing these periodic arrays of leucine residues were proposed to exist in an alpha-helical conformation and the leucine side chains from one alpha helix interdigitate with those from the alpha helix of a second polypeptide, facilitating dimerization.
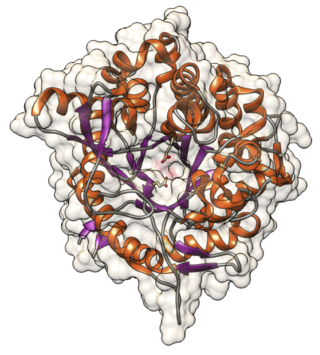
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. The receptors are generally activated by dimerization and substrate presentation. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.
The ErbB family of proteins contains four receptor tyrosine kinases, structurally related to the epidermal growth factor receptor (EGFR), its first discovered member. In humans, the family includes Her1, Her2 (ErbB2), Her3 (ErbB3), and Her4 (ErbB4). The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease, while excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor.
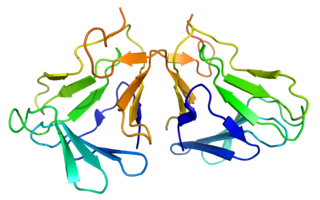
Curculin or neoculin is a sweet protein that was discovered and isolated in 1990 from the fruit of Curculigo latifolia (Hypoxidaceae). Like miraculin, curculin exhibits taste-modifying activity; however, unlike miraculin, it also exhibits a sweet taste by itself. After consumption of curculin, water and sour solutions taste sweet.
The IκB kinase is an enzyme complex that is involved in propagating the cellular response to inflammation, specifically the regulation of lymphocytes.

Receptor tyrosine-protein kinase erbB-3, also known as HER3, is a membrane bound protein that in humans is encoded by the ERBB3 gene.

Activin and inhibin are two closely related protein complexes that have almost directly opposite biological effects. Identified in 1986, activin enhances FSH biosynthesis and secretion, and participates in the regulation of the menstrual cycle. Many other functions have been found to be exerted by activin, including roles in cell proliferation, differentiation, apoptosis, metabolism, homeostasis, immune response, wound repair, and endocrine function. Conversely, inhibin downregulates FSH synthesis and inhibits FSH secretion. The existence of inhibin was hypothesized as early as 1916; however, it was not demonstrated to exist until Neena Schwartz and Cornelia Channing's work in the mid-1970s, after which both proteins were molecularly characterized ten years later.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
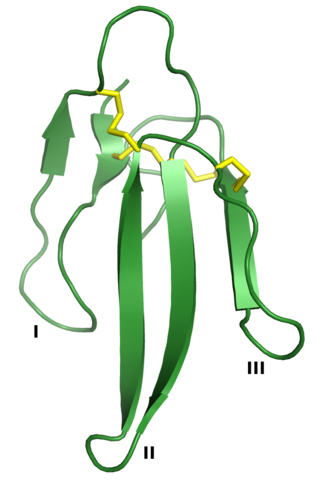
Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).
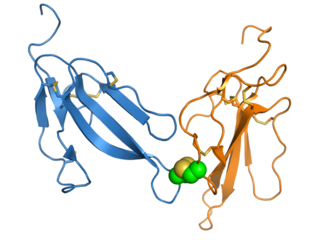
Irditoxin is a three-finger toxin (3FTx) protein found in the venom of the brown tree snake and likely in other members of the genus Boiga. It is a heterodimer composed of two distinct protein chains, each of the three-finger protein fold, linked by an intermolecular disulfide bond. This structure is unusual for 3FTx proteins, which are most commonly monomeric.
6. Conn. (2013). G protein coupled receptors modeling, activation, interactions and virtual screening (1st ed.). Academic Press.
7. Matthews, Jacqueline M. Protein Dimerization and Oligomerization in Biology. Springer New York, 2012.
8. Hjorleifsson, Jens Gu[eth]Mundur, and Bjarni Asgeirsson. “Cold-Active Alkaline Phosphatase Is Irreversibly Transformed into an Inactive Dimer by Low Urea Concentrations.” Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, vol. 1864, no. 7, 2016, pp. 755–765, https://doi.org/10.1016/j.bbapap.2016.03.016.














